ASTM F1350-24
(Specification)Standard Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Surgical Fixation Wire (UNS S31673)
Standard Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Surgical Fixation Wire (UNS S31673)
ABSTRACT
This specification covers UNS S31673 chromium-nickel-molybdenum wrought annealed stainless steel surgical fixation wires. The wires should conform to the required values of tensile strength and elongation. Wire surfaces should be processed to minimize tool marks, nicks, scratches, cracks, cavities, spurs, and other imperfections that would impair wire serviceability.
SCOPE
1.1 This specification covers the chemical, mechanical, and metallurgical requirements for the manufacture of wrought 18chromium-14nickel-2.5molybdenum stainless steel in the form of surgical fixation wire.
1.2 Units—The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system are not necessarily exact equivalents; therefore, to ensure conformance with the standard, each system shall be used independently of the other, and values from the two systems shall not be combined.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F1350 − 24
Standard Specification for
Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless
1
Steel Surgical Fixation Wire (UNS S31673)
This standard is issued under the fixed designation F1350; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* lic Surgical Implants
F138 Specification for Wrought 18Chromium-14Nickel-
1.1 This specification covers the chemical, mechanical, and
2.5Molybdenum Stainless Steel Bar and Wire for Surgical
metallurgical requirements for the manufacture of wrought
Implants (UNS S31673)
18chromium-14nickel-2.5molybdenum stainless steel in the
F981 Practice for Assessment of Muscle and Bone Tissue
form of surgical fixation wire.
Responses to Long-Term Implantable Materials Used in
1.2 Units—The values stated in either SI units or inch-
Medical Devices
pound units are to be regarded separately as standard. The
IEEE/ASTM SI 10 American National Standard for Metric
values stated in each system are not necessarily exact equiva-
Practice
lents; therefore, to ensure conformance with the standard, each 3
2.2 USP Standard:
system shall be used independently of the other, and values
U.S. Pharmacopeia Nonabsorbable Surgical Suture
from the two systems shall not be combined.
4
2.3 ISO Standards:
1.3 This standard does not purport to address all of the
ISO 9001 Quality Management System—Requirements
safety concerns, if any, associated with its use. It is the
ISO 13485 Quality Management Systems—Requirements
responsibility of the user of this standard to establish appro-
for Regulatory Purposes
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use. 3. Terminology
1.4 This international standard was developed in accor-
3.1 Definitions of Terms Specific to This Standard:
dance with internationally recognized principles on standard-
3.1.1 lot, n—the total number of mill products produced
ization established in the Decision on Principles for the
from the same melt heat under the same conditions at essen-
Development of International Standards, Guides and Recom-
tially the same time.
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
4. General Requirements for Delivery
4.1 In addition to the requirements of this specification, all
2. Referenced Documents
requirements of the current editions of Specifications A555/
2
2.1 ASTM Standards:
A555M and F138 apply.
A555/A555M Specification for General Requirements for
4.2 In cases where a conflict exists between this specifica-
Stainless Steel Wire and Wire Rods
tion and the standards listed in Section 2, this specification
E8/E8M Test Methods for Tension Testing of Metallic Ma-
shall take precedence.
terials
E29 Practice for Using Significant Digits in Test Data to
5. Ordering Information
Determine Conformance with Specifications
5.1 Inquiries and orders for material under this specification
F86 Practice for Surface Preparation and Marking of Metal-
shall include the following information:
5.1.1 Quantity,
1
This specification is under the jurisdiction of ASTM Committee F04 on
5.1.2 ASTM designation and date of issue,
Medical and Surgical Materials and Devices and is the direct responsibility of
5.1.3 Material requirements,
Subcommittee F04.12 on Metallurgical Materials.
5.1.4 Mechanical properties,
Current edition approved March 15, 2024. Published April 2024. Originally
approved in 1991. Last previous edition approved in 2015 as F1350 – 15. DOI:
10.1520/F1350-24.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available from U.S. Pharmacopeia (USP), 12601 Twinbrook Pkwy., Rockville,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM MD 20852-1790, http://www.usp.org.
4
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1350 − 24
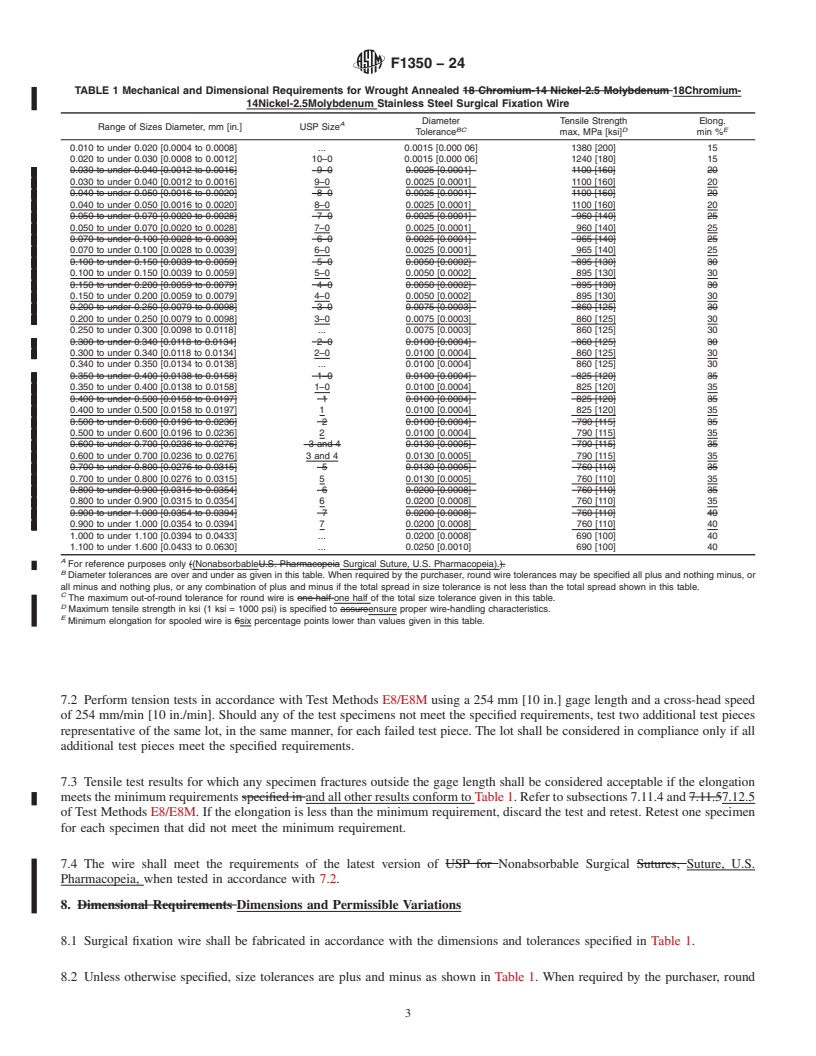
5.1.5 Form, results conform to Table 1. Refer to subsections 7.11.4 and
5.1.6 Dimensional requirements, including diameter and 7.12.5 of Test Methods E8/E8M. If the elongation is less than
diame
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F1350 − 15 F1350 − 24
Standard Specification for
Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless
1
Steel Surgical Fixation Wire (UNS S31673)
This standard is issued under the fixed designation F1350; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope*
1.1 This specification covers the chemical, mechanical, and metallurgical requirements for the manufacture of wrought 18
chromium-14 nickel-2.5 molybdenum 18chromium-14nickel-2.5molybdenum stainless steel in the form of surgical fixation wire.
1.2 Units—The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in
each system may not beare not necessarily exact equivalents; therefore, to ensure conformance with the standard, each system shall
be used independently of the other. Combiningother, and values from the two systems may result in non-conformance with this
specification.shall not be combined.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
A555/A555M Specification for General Requirements for Stainless Steel Wire and Wire Rods
E8/E8M Test Methods for Tension Testing of Metallic Materials
E29 Practice for Using Significant Digits in Test Data to Determine Conformance with Specifications
F86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
F138 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants
(UNS S31673)
F981 Practice for Assessment of Muscle and Bone Tissue Responses to Long-Term Implantable Materials Used in Medical
Devices
IEEE/ASTM SI 10 American National Standard for Metric Practice
3
2.2 USP Standard:
Nonabsorbable Surgical Suture, U.S. Pharmacopeia Nonabsorbable Surgical Suture
1
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.12 on Metallurgical Materials.
Current edition approved May 1, 2015March 15, 2024. Published June 2015April 2024. Originally approved in 1991. Last previous edition approved in 20082015 as
F1350 – 08.F1350 – 15. DOI: 10.1520/F1350-15.10.1520/F1350-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from U.S. Pharmacopeia (USP), 12601 Twinbrook Pkwy., Rockville, MD 20852-1790, http://www.usp.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1350 − 24
4
2.3 ISO Standard:Standards:
ISO 9001 Quality Management System—Requirements
ISO 13485 Quality Management Systems—Requirements for Regulatory Purposes
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 lot, n—the total number of mill products produced from the same melt heat under the same conditions at essentially the same
time.
4. General Requirements for Delivery
4.1 In addition to the requirements of this specification, all requirements of the current editions of Specifications A555/A555M
and F138 apply.
4.2 In cases where a conflict exists between this specification and the standards listed in Section 2, this specification shall take
precedence.
4. Terminology
4.1 Definitions of Terms Specific to This Standard:
4.1.1 lot, n—the total number of mill products produced from the same melt heat under the same conditions at essentially the same
time.
5. Ordering Information
5.1 Inquiries and orders
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.