ASTM D4129-05(2020)
(Test Method)Standard Test Method for Total and Organic Carbon in Water by High Temperature Oxidation and by Coulometric Detection
Standard Test Method for Total and Organic Carbon in Water by High Temperature Oxidation and by Coulometric Detection
SIGNIFICANCE AND USE
5.1 This test method is necessary because of the need for rapid reliable tests for carbonaceous material in waters and sediments.
5.2 It is used for determining the concentration of organic carbon in water that comes from a variety of natural, domestic, and industrial sources. Typically, these measurements are used to monitor organic pollutants in domestic and industrial waste water.
5.3 When a sample is homogenized so that particulate, immiscible phases, and dissolved carbon from both organic and inorganic sources is determined, the measurement is called total carbon (TC). When inorganic carbon response is eliminated by removing the dissolved CO2 prior to the analysis or the dissolved CO2 concentration subtracted from the total carbon concentration, the measurement is called total organic carbon (TOC). When particulates and immiscible phases are removed prior to analysis the measurement is called dissolved carbon (DC), or dissolved organic carbon (DOC) if inorganic carbon response has been eliminated.
5.4 Homogenizing or sparging of a sample, or both, may cause loss of volatile organics, thus yielding a negative error. The extent and significance of such losses must be evaluated on an individual basis. If significant quantities of volatile carbonaceous materials are present or may be present in samples organic carbon should be determined by the difference between the total carbon and the inorganic carbon concentrations. When organic carbon determined both by difference and by sparging agree it is acceptable to determine organic carbon by sparging for similar samples.
5.5 The relationship of TOC to other water quality parameters such as COD and BOD is described in the literature.5
SCOPE
1.1 This test method covers the determination of total and organic carbon in water and waste water, including brackish waters and brines in the range from 2 to 20 000 mg/L. This test method has the advantages of a wide range of concentration which may be determined without sample dilution and the provision for boat or capillary introduction of samples containing sediments and particulate matter where syringe injection is inappropriate.
1.2 This procedure is applicable only to that carbonaceous matter in the sample that can be introduced into the reaction zone. When syringe injection is used to introduce samples into the combustion zone, the syringe needle opening size limits the maximum size of particles that can be present in samples. Sludge and sediment samples must be homogenized prior to sampling with a micropipetor or other appropriate sampler and ladle introduction into the combustion zone is required.
1.3 The precision and bias information reported in this test method was obtained in collaborative testing that included waters of the following types: distilled, deionized, potable, natural, brine, municipal and industrial waste, and water derived from oil shale retorting. Since the precision and bias information reported may not apply to waters of all matrices, it is the user’s responsibility to ensure the validity of this test method on samples of other matrices.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific precautionary statements, see 9.1 and 10.2.1.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D4129 − 05 (Reapproved 2020)
Standard Test Method for
Total and Organic Carbon in Water by High Temperature
Oxidation and by Coulometric Detection
This standard is issued under the fixed designation D4129; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.6 This international standard was developed in accor-
dance with internationally recognized principles on standard-
1.1 This test method covers the determination of total and
ization established in the Decision on Principles for the
organic carbon in water and waste water, including brackish
Development of International Standards, Guides and Recom-
watersandbrinesintherangefrom2to20 000mg/L.Thistest
mendations issued by the World Trade Organization Technical
method has the advantages of a wide range of concentration
Barriers to Trade (TBT) Committee.
which may be determined without sample dilution and the
provision for boat or capillary introduction of samples contain-
2. Referenced Documents
ing sediments and particulate matter where syringe injection is
2.1 ASTM Standards:
inappropriate.
D513 Test Methods for Total and Dissolved Carbon Dioxide
1.2 This procedure is applicable only to that carbonaceous
in Water
matter in the sample that can be introduced into the reaction
D1129 Terminology Relating to Water
zone. When syringe injection is used to introduce samples into
D1193 Specification for Reagent Water
thecombustionzone,thesyringeneedleopeningsizelimitsthe
D3370 Practices for Sampling Water from Flowing Process
maximum size of particles that can be present in samples.
Streams
Sludge and sediment samples must be homogenized prior to
D3856 Guide for Management Systems in Laboratories
sampling with a micropipetor or other appropriate sampler and
Engaged in Analysis of Water
ladle introduction into the combustion zone is required.
D4210 Practice for Intralaboratory Quality Control Proce-
1.3 The precision and bias information reported in this test
dures and a Discussion on Reporting Low-Level Data
method was obtained in collaborative testing that included
(Withdrawn 2002)
waters of the following types: distilled, deionized, potable,
D5789 Practice for Writing Quality Control Specifications
natural, brine, municipal and industrial waste, and water
for Standard Test Methods for Organic Constituents
derived from oil shale retorting. Since the precision and bias
(Withdrawn 2002)
information reported may not apply to waters of all matrices, it
3. Terminology
is the user’s responsibility to ensure the validity of this test
method on samples of other matrices.
3.1 Definitions:
3.1.1 For definitions of terms used in this standard, refer to
1.4 The values stated in SI units are to be regarded as
Terminology D1129.
standard. No other units of measurement are included in this
standard.
4. Summary of Test Method
1.5 This standard does not purport to address all of the
4.1 The sample is homogenized or diluted, or both, as
safety concerns, if any, associated with its use. It is the
necessary. If the sample does not contain suspended particles
responsibility of the user of this standard to establish appro-
or high-salt level a 0.200-mL portion is injected into the
priate safety, health, and environmental practices and deter-
reactionzone.Forsamplescontainingsolidsorhighsaltlevels,
mine the applicability of regulatory limitations prior to use.
portions are placed in combustion boats containing tungsten
For specific precautionary statements, see 9.1 and 10.2.1.
trioxide (WO ) or quartz capillaries and introduced into the
1 2
This test method is under the jurisdiction of ASTM Committee D19 on Water For referenced ASTM standards, visit the ASTM website, www.astm.org, or
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Jan. 1, 2020. Published January 2020. Originally the ASTM website.
approved in 1982. Last previous edition approved in 2013 as D4129 – 05 (2013). The last approved version of this historical standard is referenced on
DOI: 10.1520/D4129-05R20. www.astm.org.
Copyright ©ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA19428-2959. United States
D4129 − 05 (2020)
reaction zone using a ladle. In the reaction zone the heat, an individual basis. If significant quantities of volatile carbo-
oxidation catalyst and oxygen atmosphere convert carbona- naceous materials are present or may be present in samples
ceous matter to carbon dioxide (CO ). The oxygen gas stream organiccarbonshouldbedeterminedbythedifferencebetween
sweeps the gaseous reaction products through a series of thetotalcarbonandtheinorganiccarbonconcentrations.When
scrubbers for potentially interfering gases and then to the organic carbon determined both by difference and by sparging
absorption/titration cell. The CO is determined by automatic agree it is acceptable to determine organic carbon by sparging
coulometric titration. Calibration by testing known carbon for similar samples.
content standards is not required, however, standards are
5.5 The relationship of TOC to other water quality param-
analyzed periodically to confirm proper operation. 5
eters such as COD and BOD is described in the literature.
4.2 Carbon dioxide is liberated from carbonates as well as
6. Interferences
from organic matter under the reaction conditions. Organic
6.1 Any acidic or basic gas formed in the oxidation of the
carbon is determined by difference between the total carbon
sample and not removed by the scrubbers will interfere with
and the inorganic carbon determined separately or by acidify-
the test. Potentially interfering gases that are removed by the
ing a portion of the sample to a pH of 2 or less and sparging
scrubbers include hydrogen sulfide (H S), hydrogen chloride
with carbon dioxide-free gas to remove carbonates,
(HCl), hydrogen bromide (HBr), hydrogen iodide (HI), sulfur
bicarbonates, and dissolved carbon dioxide prior to total
dioxide (SO ), sulfur trioxide (SO ) free halogens, halogen
carbon determination. To determine organic carbon by differ- 2 3
oxides, and nitrogen oxides. Hydrogen fluoride (HF) may be
ence the inorganic carbon is determined by acid release of
removedbybubblingthegasstreamthroughwaterinthewater
carbon dioxide from a portion of the sample or other methods
vapor condenser.
as given in Test Methods D513. For discussion of the limita-
tions and guidelines for the use of the sparge technique, see 5.4
6.2 The capacity of the scrubbers for potentially interfering
and the paper by Van Hall.
gases may vary with the type of samples being analyzed. If the
scrubber capacity is exceeded it can be recognized by the
4.3 Because of the various properties of carbon-containing
titrationcontinuingbeyondthenormalanalysistimeatahigher
compounds in water, any preliminary treatment of a sample
rate than the blank and high results for known carbon content
prior to injection dictates a definition of the carbon measured.
standards as well as by appearance changes in the scrubbers. If
Filtration of the sample prior to injection will limit the carbon
the scrubber capacity is exceeded during an analysis the
measuredtodissolvedcarbonatesanddissolvedorganicmatter.
scrubbers should be replaced and the analysis repeated.
Homogenizing permits determination of the carbon in in-
Samples containing all concentrations of the potentially inter-
soluble carbonates and insoluble organic materials.
fering species can be analyzed if the analyst uses great care to
5. Significance and Use
ensure that the scrubbers are and remain effective for his
samples. The frequency of replacing the scrubbers will depend
5.1 This test method is necessary because of the need for
on the nature of the samples.
rapid reliable tests for carbonaceous material in waters and
sediments.
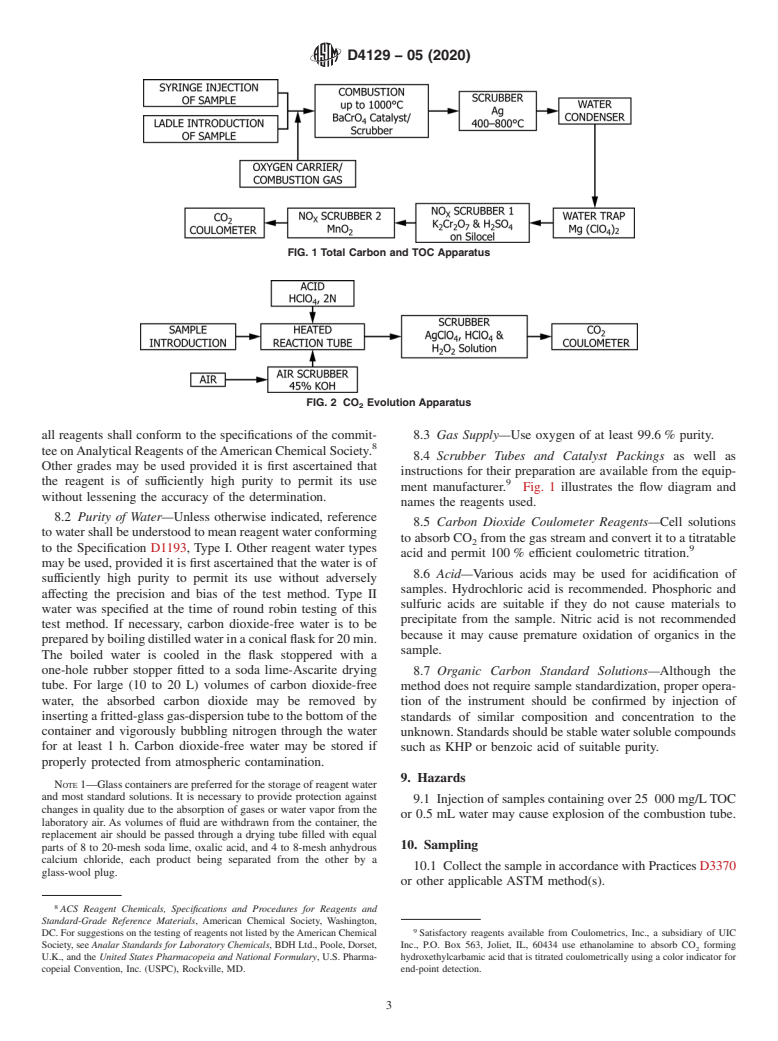
7. Apparatus
5.2 It is used for determining the concentration of organic
7.1 Apparatus for total carbon, organic carbon, and inor-
carbon in water that comes from a variety of natural, domestic,
ganic carbon determinations-combustion furnace with gas
and industrial sources. Typically, these measurements are used
supply, gas purification train, flow control, acid reaction train,
to monitor organic pollutants in domestic and industrial waste
and carbon dioxide coulometer. Fig. 1 and Fig. 2 show block
water.
diagrams of the apparatus.
5.3 When a sample is homogenized so that particulate,
7.2 Sampling Devices—A spring-loaded 0.200-mL syringe
immiscible phases, and dissolved carbon from both organic
(carbon analyzer syringe) having an all metal tip and a 50 mm
and inorganic sources is determined, the measurement is called
long, 0.5-mm inside diameter needle with a square end is
total carbon (TC). When inorganic carbon response is elimi-
recommended for water samples containing little or no particu-
nated by removing the dissolved CO prior to the analysis or
late matter.
the dissolved CO concentration subtracted from the total
7.3 Homogenizing Apparatus—A household blender with
carbon concentration, the measurement is called total organic
glass mixing chamber is generally satisfactory for homogeniz-
carbon (TOC). When particulates and immiscible phases are
ing immiscible phases in water.
removed prior to analysis the measurement is called dissolved
carbon (DC), or dissolved organic carbon (DOC) if inorganic
8. Reagents
carbon response has been eliminated.
8.1 Purity of Reagents—Reagent grade chemicals shall be
5.4 Homogenizing or sparging of a sample, or both, may
used in all tests. Unless otherwise indicated, it is intended that
cause loss of volatile organics, thus yielding a negative error.
Theextentandsignificanceofsuchlossesmustbeevaluatedon
Handbook for Monitoring Industrial Wastewater, U.S. Environment Protection
Agency, August 1973, pp. 5-10 to 5-12.
Instruments marketed by Coulometrics, Inc., a subsidiary of UIC Inc., P.O. Box
Van Hall, C. E., Barth, D., and Stenger, V.A., “Elimination of Carbonates from 563, Joliet, IL, 60434, or an equivalent, have been found satisfactory.
Aqueous Solutions Prior to Organic Carbon Determinations,” Analytical Chemistry, Syringes manufactured by Hamilton Co., P.O. Box 10030, Reno, NV 89510, or
Vol 37, 1965, pp. 769–771. an equivalent, have been found satisfactory for this purpose.
D4129 − 05 (2020)
FIG. 1 Total Carbon and TOC Apparatus
FIG. 2 CO Evolution Apparatus
all reagents shall conform to the specifications of the commit- 8.3 Gas Supply—Use oxygen of at least 99.6 % purity.
tee onAnalytical Reagents of theAmerican Chemical Society.
8.4 Scrubber Tubes and Catalyst Packings as well as
Other grades may be used provided it is first ascertained that
instructions for their preparation are available from the equip-
the reagent is of sufficiently high purity to permit its use 9
ment manufacturer. Fig. 1 illustrates the flow diagram and
without lessening the accuracy of the determination.
names the reagents used.
8.2 Purity of Water—Unless otherwise indicated, reference
8.5 Carbon Dioxide Coulometer Reagents—Cell solutions
to water shall be understood to mean reagent water conforming
to absorb CO from the gas stream and convert it to a titratable
to the Specification D1193, Type I. Other reagent water types 9
acid and permit 100 % efficient coulometric titration.
may be used, provided it is first ascertained that the water is of
8.6 Acid—Various acids may be used for acidification of
sufficiently high purity to permit its use without adversely
samples. Hydrochloric acid is recommended. Phosphoric and
affecting the precision and bias of the test method. Type II
sulfuric acids are suitable if they do not cause materials to
water was specified at the time of round robin testing of this
precipitate from the sample. Nitric acid is not recommended
test method. If necessary, carbon dioxide-free water is to be
because it may cause premature oxidation of organics in the
preparedbyboilingdistilledwaterinaconicalflaskfor20min.
sample.
The boiled water is cooled in the flask stoppered with a
one-hole rubber stopper fitted to a soda lime-Ascarite drying 8.7 Organic Carbon Standard Solutions—Although the
tube. For large (10 to 20 L) volumes of carbon dioxide-free
method does not require sample standardization, proper opera-
water, the absorbed carbon dioxide may be removed by tion of the instrument should be confirmed by injection of
insertingafritted-glassgas-dispersiontubetothebottomofthe
standards of similar composition and concentration to the
container and vigorously bubbling nitrogen through the water
unknown.Standardsshouldbestablewatersolublecompounds
for at least 1 h. Carbon dioxide-free water may be stored if such as KHP or benzoic acid of suitable purity.
properly protected from atmospheric contamination.
9. Hazards
NOTE 1—Glass containers are preferred for the storage of reagent water
and most standard solutions. It is necessary to provide protection against
9.1 Injection of samples containing over 25 000 mg/LTOC
changes in quality due to the absorption of gases or water vapor from the
or 0.5 mL water may cause explosion of the combustion tube.
laboratory air. As volumes of fluid are withdrawn from the container, the
replacement air should be passed through a drying tube filled with equal
10. Sampling
parts of 8 to 20-mesh soda lime, oxalic acid, and 4 to 8-mesh anhydrous
calcium chloride, each product being separated from the other by a
10.1 Collect the sample in accordance with PracticesD3370
glass-wool plug.
or other applicable ASTM method(s).
ACS Reagent Chemicals, Specifications and Procedures for Reagents and
Standard-Grade Reference Materials, American Chemical Society, Washington,
DC. For suggestions on the testing of reagents not listed by theAmerican Chemical Satisfactory reagents available from Coulometrics, Inc., a subsidiary of UIC
Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, Inc., P.O. Box 563, Joliet, IL, 60434 use ethanolamine to absorb CO forming
U.K., and the United States Pharmacopeia and National Formulary, U.S. Pharma- hydroxethylcarbamic acid that is titrated coulometrically using a color indicator for
copeial Con
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.