ASTM F1876-98(2003)e1
(Specification)Standard Specification for Polyetherketoneetherketoneketone (PEKEKK) Resins for Surgical Implant Applications (Withdrawn 2012)
Standard Specification for Polyetherketoneetherketoneketone (PEKEKK) Resins for Surgical Implant Applications (Withdrawn 2012)
ABSTRACT
This specification covers polyetherketoneetherketoneketone (PEKEKK) resins in virgin forms as supplied by a vendor, such as flakes, pellets, blocks, and so forth. It provides requirements and associated test methods for these thermoplastics when they are to be used in the manufacturing of intracorporeal devices, such as surgical implants or components of surgical or dental devices. The properties of fabricated forms of these resins should be evaluated using test methods that are appropriate to assure safety and efficacy. PEKEKK resins in the scope of this specification are pure semicrystalline homopolymers consisting of phenylene rings connected by either (E) and carbonyl (or ketone, K) groups along the polymer chain.
SCOPE
1.1 This specification covers polyetherketoneetherketoneketone (PEKEKK) resins in virgin forms as supplied by a vendor, such as flakes, pellets, blocks, etc. It provides requirements and associated test methods for these thermoplastics when they are to be used in the manufacturing of intracorporeal devices, such as surgical implants or components of surgical or dental devices.
1.2 As with any material, some characteristics may be altered by the processing techniques, such as molding, extrusion, machining, assembly, sterilization, etc. required for the production of a specific part or device; therefore, properties of fabricated forms of these resins should be evaluated using test methods that are appropriate to assure safety and efficacy as agreed upon by the vendor, purchaser, and regulating bodies.
1.3 The properties included in this specification are those applicable for PEKEKK resins only. Fabricated forms, material or forms containing colorants, fillers, processing aids, or other additives, as well as polymer blends tht contain PEKEKK, are not covered by this specification.
1.4 This specification is designed to recommend physical, chemical, and biological test methods to establish a reasonable level of confidence concerning the performance of virgin PEKEKK resins for use in medical devices. The properties listed should be considered in selecting material according to the specific end-use requirement.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
WITHDRAWN RATIONALE
This specification covers polyetherketoneetherketoneketone (PEKEKK) resins in virgin forms as supplied by a vendor, such as flakes, pellets, blocks, and so forth. It provides requirements and associated test methods for these thermoplastics when they are to be used in the manufacturing of intracorporeal devices, such as surgical implants or components of surgical or dental devices.
Formerly under the jurisdiction of Committee F04 on Medical and Surgical Materials and Devices, this specification was withdrawn in January 2012 in accordance with section 10.5.3.1 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: F1876 – 98 (Reapproved 2003)
Standard Specification for
Polyetherketoneetherketoneketone (PEKEKK) Resins for
Surgical Implant Applications
This standard is issued under the fixed designation F1876; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Table 2 was editorially corrected in June 2003.
1. Scope 2. Referenced Documents
1.1 Thisspecificationcoverspolyetherketoneetherketoneke- 2.1 ASTM Standards:
tone (PEKEKK) resins in virgin forms as supplied by a vendor, D149 Test Method for Dielectric Breakdown Voltage and
such as flakes, pellets, blocks, and so forth. It provides Dielectric Strength of Solid Electrical Insulating Materials
requirementsandassociatedtestmethodsforthesethermoplas- at Commercial Power Frequencies
tics when they are to be used in the manufacturing of D256 Test Methods for Determining the Izod Pendulum
intracorporeal devices, such as surgical implants or compo- Impact Resistance of Plastics
nents of surgical or dental devices. D570 Test Method for Water Absorption of Plastics
1.2 As with any material, some characteristics may be D638 Test Method for Tensile Properties of Plastics
altered by the processing techniques, such as molding, extru- D648 Test Method for Deflection Temperature of Plastics
sion, machining, assembly, sterilization, and so forth required Under Flexural Load in the Edgewise Position
for the production of a specific part or device; therefore, D695 Test Method for Compressive Properties of Rigid
properties of fabricated forms of these resins should be Plastics
evaluated using test methods that are appropriate to assure D696 Test Method for Coefficient of Linear Thermal Ex-
safety and efficacy as agreed upon by the vendor, purchaser, pansion of Plastics Between Ø30°C and 30°C with a
and regulating bodies. Vitreous Silica Dilatometer
1.3 The properties included in this specification are those D790 Test Methods for Flexural Properties of Unreinforced
applicableforPEKEKKresinsonly.Fabricatedforms,material and Reinforced Plastics and Electrical Insulating Materials
or forms containing colorants, fillers, processing aids, or other D792 Test Methods for Density and Specific Gravity (Rela-
additives, as well as polymer blends that contain PEKEKK, are tive Density) of Plastics by Displacement
not covered by this specification. D955 Test Method of Measuring Shrinkage from Mold
1.4 This specification is designed to recommend physical, Dimensions of Thermoplastics
chemical, and biological test methods to establish a reasonable D1238 Test Method for Melt Flow Rates of Thermoplastics
level of confidence concerning the performance of virgin by Extrusion Plastometer
PEKEKK resins for use in medical devices. The properties D1505 Test Method for Density of Plastics by the Density-
listed should be considered in selecting material according to Gradient Technique
the specific end-use requirements. D1898 Practice for Sampling of Plastics
1.5 This standard does not purport to address all of the D3417 Test Method for Enthalpies of Fusion and Crystal-
safety concerns, if any, associated with its use. It is the lization of Polymers by Differential Scanning Calorimetry
responsibility of the user of this standard to establish appro- (DSC)
priate safety and health practices and determine the applica- D3418 Test Method for Transition Temperatures and En-
bility of regulatory limitations prior to use. thalpies of Fusion and Crystallization of Polymers by
1 2
This specification is under the jurisdiction of ASTM Committee F04 on For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Medical and Surgical Materials and Devices and is the direct responsibility of contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Subcommittee F04.11 on Polymeric Materials. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Apr. 10, 2003. Published June 2003. Originally the ASTM website.
approved in 1998. Last previous edition approved in 1998 as F1876 – 98. DOI: Withdrawn. The last approved version of this historical standard is referenced
10.1520/F1876-98R03E01. on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
F1876 – 98 (2003)
5. Properties
5.1 PEKEKK resins used in medical applications may
comply with the Food and Drug Administration (FDA) Regu-
lation 21 CFR 177.1580, which covers both wet and dry food
contact applications.
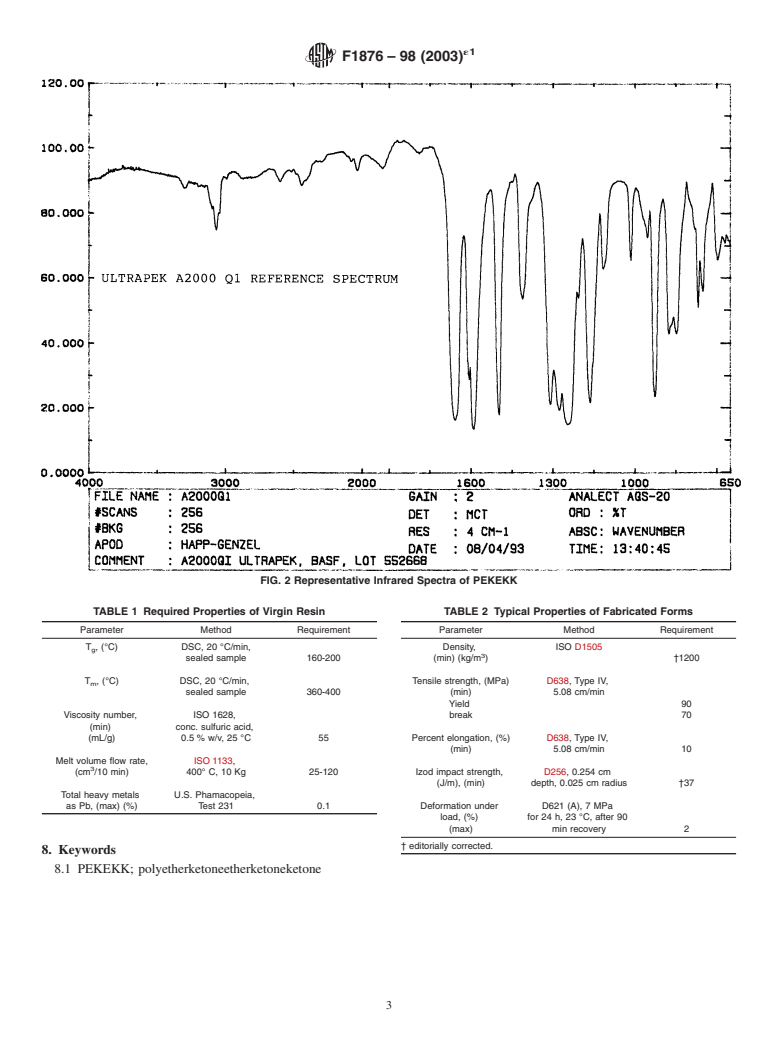
5.2 The infrared spectrum (1) of these materials is charac-
FIG. 1 Chemical Structure of PEKEKK
teristic of their molecular repeating units. A representative
spectrum is provided in Fig. 2. The PEKEKK resin shall yield
Differential Scanning Calorimetry
an infrared transmittance spectrum that exhibits major bands
D4000 Classification System for Specifying Plastic Materi-
only at the wavelengths listed for a standard reference spec-
als
trum of that material.
F748 Practice for Selecting Generic Biological Test Meth-
5.2.1 The infrared spectrum, as used herein, is to identify
ods for Materials and Devices
the specific type of PAEK present and does not necessarily
2.2 ISO Standards:
indicate an acceptable degree of material purity.
ISO 1628/1 Plastics–Guidelines for the Standardization of
5.2.2 The presence of additional bands in the sample’s
Methods for Determination of Viscosity Number and
infrared spectrum compared to that of the reference material
Limiting Viscosity Number of Polymers in Dilute Solu-
may indicate a different PAEK, impurities, or both.
tion–Part 1: General Conditions
5.3 Thephysicalandchemicalpropertyrequirementsforthe
ISO 1133 Plastics–Determination of the Melt Mass-Flow
virgin resin are listed in Table 1. If additional characteristics
Rate (MFR) and the Melt Volume-Flow Rate (MVR) of
are necessary because of a specific application, the procedures
Thermoplastics
referenced in 5.7 are recommended, or as agreed upon by
ISO 10993 Biological Evaluation of Medical Devices, Parts
vendor and purchaser.
1–12
5.4 The solution viscosity requirements listed in Table 1
2.3 Other Documents:
may be supplemented, or replaced, by rheological or complex
FDA Regulation CFR 177.1580
viscosity data as agreed upon by vendor and purchaser.
United States Pharmacopeia, Vol XXI, or latest edition
5.5 The chemical, physical, and mechanical properties of
3. Terminology
fabricated forms are related to the processes utilized in
producing the fabricated form, for example, molding, machin-
3.1 Definitions of Terms Specific to This Standard:
ing, sterilization, and so forth. Additionally, the properties
3.1.1 fabricated forms, n—those items into which the virgin
necessary for a particular device to perform properly will vary
forms may be converted. These forms include shapes and
from one device type to another. Table 2 lists some typical
forms produced by means of machining, extruding, and com-
properties of nonsterilized injection molded material.
pression molding virgin forms into a subsequent entity, for
5.6 Test specim
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.