ASTM F1876-98
(Specification)Standard Specification for Polyetherketoneetherketoneketone (PEKEKK) Resins for Surgical Implant Applications
Standard Specification for Polyetherketoneetherketoneketone (PEKEKK) Resins for Surgical Implant Applications
SCOPE
1.1 This specification covers polyetherketoneetherketoneketone (PEKEKK) resins in virgin forms as supplied by a vendor, such as flakes, pellets, blocks, etc. It provides requirements and associated test methods for these thermoplastics when they are to be used in the manufacturing of intracorporeal devices, such as surgical implants or components of surgical or dental devices.
1.2 As with any material, some characteristics may be altered by the processing techniques, such as molding, extrusion, machining, assembly, sterilization, etc. required for the production of a specific part or device; therefore, properties of fabricated forms of these resins should be evaluated using test methods that are appropriate to assure safety and efficacy as agreed upon by the vendor, purchaser, and regulating bodies.
1.3 The properties included in this specification are those applicable for PEKEKK resins only. Fabricated forms, material or forms containing colorants, fillers, processing aids, or other additives, as well as polymer blends tht contain PEKEKK, are not covered by this specification.
1.4 This specification is designed to recommend physical, chemical, and biological test methods to establish a reasonable level of confidence concerning the performance of virgin PEKEKK resins for use in medical devices. The properties listed should be considered in selecting material according to the specific end-use requirement.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1876 – 98
Standard Specification for
Polyetherketoneetherketoneketone (PEKEKK) Resins for
Surgical Implant Applications
This standard is issued under the fixed designation F 1876; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 149 Test Method for Dielectric Breakdown Voltage and
Dielectric Strength of Solid Electrical Insulating Materials
1.1 This specification covers polyetherketoneetherketoneke-
at Commercial Power Frequencies
tone (PEKEKK) resins in virgin forms as supplied by a vendor,
D 256 Test Methods for Determining the Impact Resistance
such as flakes, pellets, blocks, etc. It provides requirements and
of Plastics and Electrical Insulating Materials
associated test methods for these thermoplastics when they are
D 570 Test Method for Water Absorption of Plastics
to be used in the manufacturing of intracorporeal devices, such
D 638 Test Method for Tensile Properties of Plastics
as surgical implants or components of surgical or dental
D 648 Test Method for Deflection Temperature of Plastics
devices.
Under Flexural Load
1.2 As with any material, some characteristics may be
D 695 Test Method for Compressive Properties of Rigid
altered by the processing techniques, such as molding, extru-
Plastics
sion, machining, assembly, sterilization, etc. required for the
D 696 Test Method for Coefficient of Linear Thermal Ex-
production of a specific part or device; therefore, properties of
pansion of Plastics
fabricated forms of these resins should be evaluated using test
D 790 Test Methods for Flexural Properties of Unreinforced
methods that are appropriate to assure safety and efficacy as
and Reinforced Plastics and Electrical Insulating Materi-
agreed upon by the vendor, purchaser, and regulating bodies.
als
1.3 The properties included in this specification are those
D 792 Test Methods for Relative Density, Density of Plas-
applicable for PEKEKK resins only. Fabricated forms, material
tics and Specific Gravity by Displacement
or forms containing colorants, fillers, processing aids, or other
D 955 Test Method for Measuring Shrinkage from Mold
additives, as well as polymer blends that contain PEKEKK, are
Dimensions of Molded Plastics
not covered by this specification.
D 1238 Test Method for Flow Rates of Thermoplastics by
1.4 This specification is designed to recommend physical,
Extrusion Plastometer
chemical, and biological test methods to establish a reasonable
D 1505 Test Method for Density of Plastics by the Density-
level of confidence concerning the performance of virgin
Gradient Technique
PEKEKK resins for use in medical devices. The properties
D 1898 Practice for Sampling of Plastics
listed should be considered in selecting material according to
D 3417 Test Method for Heats of Fusion and Crystallization
the specific end-use requirements.
of Polymers by Thermal Analysis
1.5 This standard does not purport to address all of the
D 3418 Test Methods for Transition Temperatures of Poly-
safety concerns, if any, associated with its use. It is the
mers by Thermal Analysis
responsibility of the user of this standard to establish appro-
D 4000 Classification System for Specifying Plastic Mate-
priate safety and health practices and determine the applica-
rials
bility of regulatory limitations prior to use.
F 748 Practice for Selecting Generic Biological Test Meth-
2. Referenced Documents ods for Materials and Devices
2.2 ISO Standards:
2.1 ASTM Standards:
ISO 1628/1 Plastics–Guidelines for the Standardization of
Methods for Determination of Viscosity Number and
1 2
This specification is under the jurisdiction of ASTM Committee F-4 on Medical Annual Book of ASTM Standards, Vol 10.01.
and Surgical Materials and Devices and is the direct responsibility of Subcommittee Annual Book of ASTM Standards, Vol 08.01.
F04.11 on Polymeric Materials. Annual Book of ASTM Standards, Vol 08.02.
Current edition approved April 10, 1998. Published June 1998. Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F1876–98
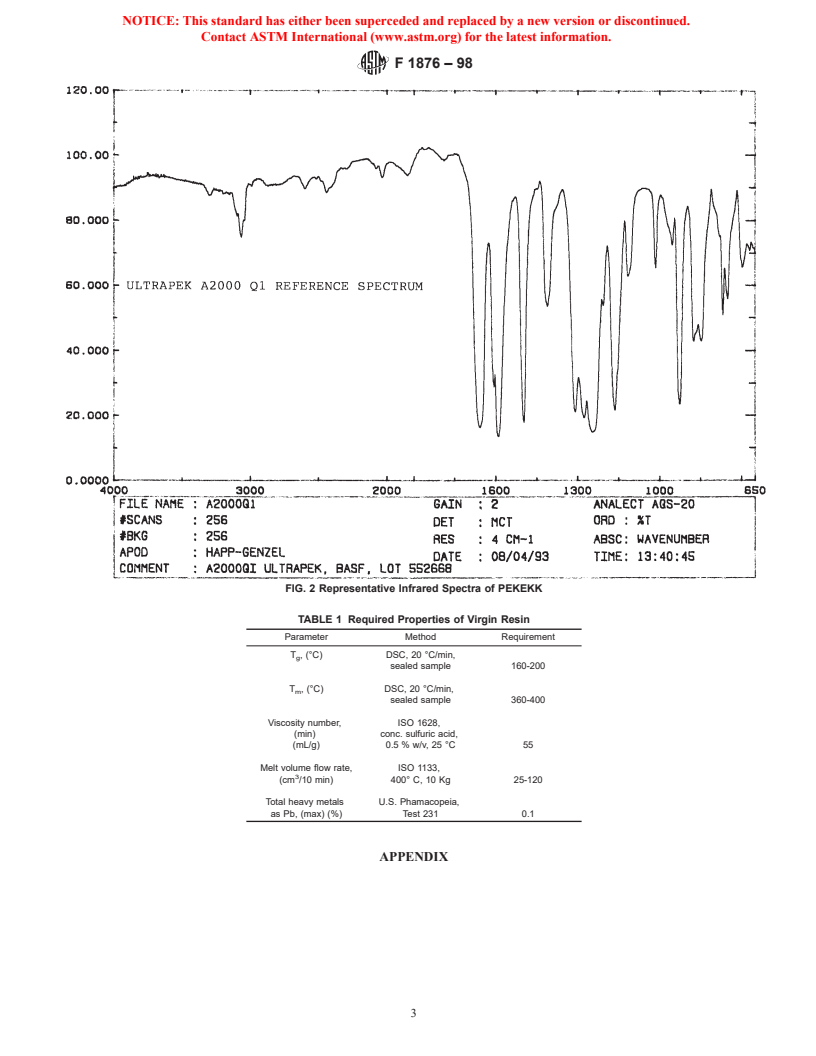
Limiting Viscosity Number of Polymers in Dilute Solu- 5.2 The infrared spectrum (1) of these materials is charac-
tion–Part 1: General Conditions teristic of their molecular repeating units. A representative
ISO 1133 Plastics–Determination of the Melt Mass-Flow spectrum is provided in Fig. 2. The PEKEKK resin shall yield
Rate (MFR) and the Melt Volume-Flow Rate (MVR) of an infrared transmittance spectrum that exhibits major bands
Thermoplastics only at the wavelengths listed for a standard reference spec-
ISO 10993 Biological Evaluation of Medical Devices, Parts trum of that material.
1–12 5.2.1 The infrared spectrum, as used herein, is to identify
2.3 Other Documents: the specific type of PAEK present and does not necessarily
FDA Regulation CFR 177.1580 indicate an acceptable degree of material purity.
United States Pharmacopeia, Vol XXI, or latest edition 5.2.2 The presence of additional bands in the sample’s
infrared spectrum compared to that of the reference material
3. Terminology
may indicate a different PAEK, impurities, or both.
3.1 Definitions of Terms Specific to This Standard:
5.3 The physical and chemical property requirements for the
3.1.1 fabricated forms, n—those items into which the virgin
virgin resin are listed in Table 1. If additional characteristics
forms may be converted. These forms include shapes and
are necessary because of a specific application, the procedures
forms produced by means of machining, extruding, and com-
referenced in 5.7 are recommended, or as agreed upon by
pression molding virgin forms into a subsequent entity, for
vendor and purchaser.
example, rods, slabs, sheets, film, complex shaped parts and
5.4 The solution viscosity requirements listed in Table 1
devices.
may be supplemented, or replaced, by rheological or complex
3.1.2 formulated compound, n—PEKEKK materials, parts,
viscosity data as agreed upon by vendor and purchaser.
or devices fabricated from virgin forms in such a way as to
5.5 The chemical, physical, and mechanical properties of
contain intentional or unintentional adjuvant substances.
fabricated forms are related to the processes utilized in
3.1.3 virgin forms, n—that form of the PEKEKK resin as
producing the fabricated form, for example, molding, machin-
obtained by the synthesizer after removal of residual mono-
ing, sterilization, etc. Additionally, the properties necessary for
mers, solvents, catalysts, etc. It typically will be in the form of
a particular device to perform properly will vary from one
pellets, chips, or blocks. It is the material from which rods,
device type to another. Table 2 lists some typical properties of
slabs, sheets, films, or specific parts and devices are fabricated.
nonsterilized injection molded material.
5.6 Test specimens shall be
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.