ASTM E1659-00
(Test Method)Standard Test Methods Coating Weight and Chemical Analysis of Zinc-Nickel Alloy Electrolytically Coated on Steel Sheet
Standard Test Methods Coating Weight and Chemical Analysis of Zinc-Nickel Alloy Electrolytically Coated on Steel Sheet
SCOPE
1.1 These test methods cover independently the chemical analysis of each surface of zinc-nickel alloy electrolytically coated on steel sheet. The coatings have chemical compositions within the following limits: Analyte Concentration Range Coating weight 0.0 to 80 g/m 2 Nickel 7.0 to 17.0%
1.2 These test methods are contained in the following sections. Sections Coating weight total, by the weigh-strip-weigh method (20.0 to 8 to 17 45.0 g/m 2 ) Nickel by the atomic absorption method (11.0 to 13.5 % of 18 to 28 coating weight ranging from 20 to 45 g/m 2 )
1.3 The values stated in SI units are to be regarded as standard. In some cases, exceptions allowed in Practice E380 are also used.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1659–00
Standard Test Methods for
Coating Weight and Chemical Analysis of Zinc-Nickel Alloy
Electrolytically Coated on Steel Sheet
This standard is issued under the fixed designation E 1659; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope tions for Chemical Analysis of Metals
E 173 Practice for Conducting Interlaboratory Studies of
1.1 These test methods cover independently the chemical
Methods for Chemical Analysis of Metals
analysis of each surface of zinc-nickel alloy electrolytically
E 380 Practice for Use of the International System of Units
coated on steel sheet. The coatings have chemical composi-
(SI) (the Modernized Metric System)
tions within the following limits:
E 663 Practice for Flame Atomic Absorption Analysis
Analyte Concentration Range
E 882 Guide for Accountability and Quality Control in the
Coating Weight 0.0 to 80 g/m
Nickel 7.0 to 17.0 %
Chemical Analysis Laboratory
E 1024 Guide for Chemical Analysis of Metals and Metal
1.2 These test methods are in the following sections:
Bearing Ores by Flame Atomic Absorption Spectropho-
Sections
Coating Weight, by the Weigh-Strip-Weigh Method 8-18 tometry
(20.0 to 45.0 g/m )
E 1452 Practice for Preparation of Calibration Solutions for
Nickel by the Atomic Absorption Method (11.0 to 13.5 % 19-29
2 Spectrophotometric and for Spectroscopic Atomic Analy-
of Coating Weight Ranging from 20 to 45 g/m )
ses
1.3 The values stated in SI units are to be regarded as
standard. In some cases, exceptions allowed in Practice E 380
3. Significance and Use
are also used.
3.1 These test methods for the chemical analysis of zinc-
1.4 This standard does not purport to address all of the
nickel alloy coating on sheet steel are primarily intended as
safety concerns, if any, associated with its use. It is the
referee methods to test such materials for compliance with
responsibility of the user of this standard to establish appro-
compositional specifications such as found in Specification
priate safety and health practices and determine the applica-
A 918, particularly those under the jurisdiction of ASTM
bility of regulatory limitations prior to use.
CommitteeA-5 on Metallic Coated Iron and Steel Products. It
is assumed that all who use these test methods will be trained
2. Referenced Documents
analysts capable of performing common laboratory procedures
2.1 ASTM Standards:
skillfully and safely. It is expected that work will be performed
A 917 Specification for Steel Sheet, Coated by the Electro-
in a properly equipped laboratory under appropriate quality
lyticProcessforApplicationsRequiringDesignationofthe
control practices such as those described in Guide E 882.
Coating Mass on Each Surface (General Requirements)
3.2 These test methods must be applied twice, once to each
A 918 Specification for Steel Sheet, Zinc-Nickel Alloy
side of the specimen if coating weight and composition are
Coated by the Electrolytic Process for Applications Re-
required for both sides of a coated sheet. Two separate
quiring Designation of the Coating Mass on Each Surface
specimens are required for this purpose.
D 1193 Specification for Reagent Water
E 29 Practice for Using Significant Digits in Test Data to 4. Apparatus, Reagents, and Instrumental Practices
Determine Conformance with Specifications
4.1 Apparatus—Specialized apparatus requirements are
E 50 Practices for Apparatus, Reagents, and Safety Precau-
listed in the apparatus section in each individual test method.
4.2 Reagents:
4.2.1 Purity of Reagents—Unless otherwise indicated, all
reagents used in these test methods shall conform to the
These test methods are under the jurisdiction of ASTM Committee E-1 on
Analytical Chemistry for Metals, Ores, and Related Materials and are the direct
responsibility of Subcommittee E01.05 on Zn, Sn, Pb, Cd, Be, and Other Metals.
Current edition approved Jan. 10, 2000. Published March 2000. Originally
published as E 1659 – 95. Last previous edition E 1659 – 95. Annual Book of ASTM Standards, Vol 03.05.
2 6
Annual Book of ASTM Standards, Vol 01.06. Discontinued; see 1997 Annual Book of ASTM Standards, Vol 03.05.
3 7
Annual Book of ASTM Standards, Vol 11.01. Discontinued; see 1997 Annual Book of ASTM Standards, Vol 03.06.
4 8
Annual Book of ASTM Standards, Vol 14.02. Annual Book of ASTM Standards, Vol 03.06.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1659
Reagent Grade Specifications of theAmerican Chemical Soci- 9. Summary of Test Method
ety. Other chemicals may be used, provided it is first ascer-
9.1 The coating on the sheet steel is stripped by using
tained that they are of sufficiently high purity to permit their
hydrochloric acid solution containing an inhibitor to prevent
use without adversely affecting the expected performance of
the attack on the base steel. The coating weight is determined
the determination, as indicated in Section 28.
from the weight difference of the specimen before and after
4.2.2 Purity of Water—References to water shall be under-
stripping.
stood to mean reagent water as defined by Type II of Specifi-
cation D 1193.
10. Interferences
4.3 Photometric Practice—Photometric practice prescribed
10.1 The hexamethylene tetramine inhibitor used in this test
in these test methods shall conform to Guide E 1024 and
method permits the dissolution of some base metal, which
Practice E 1452.
could lead to higher than expected coating weight determina-
tions. Since Zn/Ni coatings contain no appreciable amounts of
5. Hazards
Fe, the effects of this bias are corrected by determining the
5.1 For precautions to be observed in the use of certain
mass of iron stripped with the coating and subtracting that
reagentsandequipmentinthesetestmethods,refertoPractices
value from the raw weigh-strip-weigh data.
E 50.
11. Apparatus
6. Sampling
11.1 Analytical Balance, capable of weighing to 0.1 mg.
6.1 Zinc-Nickel Alloy Coated Sheets—Samples for deter-
11.2 Electroplater’s Tape, capable of protecting one side of
mining weight and composition of coating shall be secured in
a coated piece of sheet steel while the other side is being
accordance with Specification A 917, which is referred to in
stripped in a hydrochloric acid solution. It must not contami-
Specification A 918. Test specimens shall be of squares with
nate the acid solution or interfere with the coating weight
sides of 50 6 5 mm. One test specimen is required for each
determination by gaining or losing weight.
side to be analyzed. The backside which is not to be analyzed
11.3 Vernier Calipers, calibrated to an international stan-
shall be marked “X”.
dard and capable of measuring to at least 0.05 mm.
7. Interlaboratory Studies and Rounding Calculated
12. Reagents
Values
12.1 Hexamethylene Tetramine, USP Grade—Used as an
7.1 These test methods have been evaluated using Practice
inhibitor to prevent acid attack of the base metal while
E 173, except for the update in the stripping solution, 15.1,
stripping the coating from the base steel.
15.8, 18.1.2, 18.2, 29.1.1, and 29.2, as well as Tables 2, 3, 5,
12.2 Stripping Solution—Add 340 mL hydrochloric acid to
and 6.
1660 mLof water.Add 7.0 g of hexamethylene tetramine, mix,
7.2 Calculated values shall be rounded to the desired num-
and cool before use.
ber of places in accordance with the rounding method of
Practice E 29.
13. Precautions
13.1 Warning—Hydrogen gas, which can form explosive
WEIGHT OF COATING ON ZINC-NICKELALLOY-
mixtures with air, is evolved in the stripping process. There-
COATED SHEET BY WEIGH-STRIP-WEIGH
fore, this test method should be performed under conditions of
METHOD
adequate ventilation, such as a fume hood.
8. Scope
14. Sample Preparation
8.1 This test method provides a procedure for determining
14.1 Clean the specimens with acetone using a soft paper
independently the weight of coating on each surface of
towel, then dry with oil-free compressed air.
zinc-nickel alloy-coated sheet steel, in coating masses from 20
14.2 Cover the side of the specimen from which the coating
to 45 g/m (Note 1).
is not to be stripped with electroplater’s tape.
NOTE 1—The upper limit of the scope has been set at 45 g/m because
14.3 Use a roller to press the tape firmly against the sheet,
test materials with higher coating weight were not available for testing in
making sure to remove all air bubbles or wrinkles.
accordance with Practice E 173. However, recognizing the simplicity of
14.4 Trim off the excess tape.
the weigh-strip-weigh technique, materials with higher coating weights
14.5 Press the tape firmly near the edge to protect the taped
can be tested following this procedure. Users of this test method are
side from acid attack.
cautioned that use of it for coating weight determinations above 45 g/m
14.6 Write the sample identification on the taped side with
is not supported by interlaboratory testing.
a marker.
15. Procedure
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
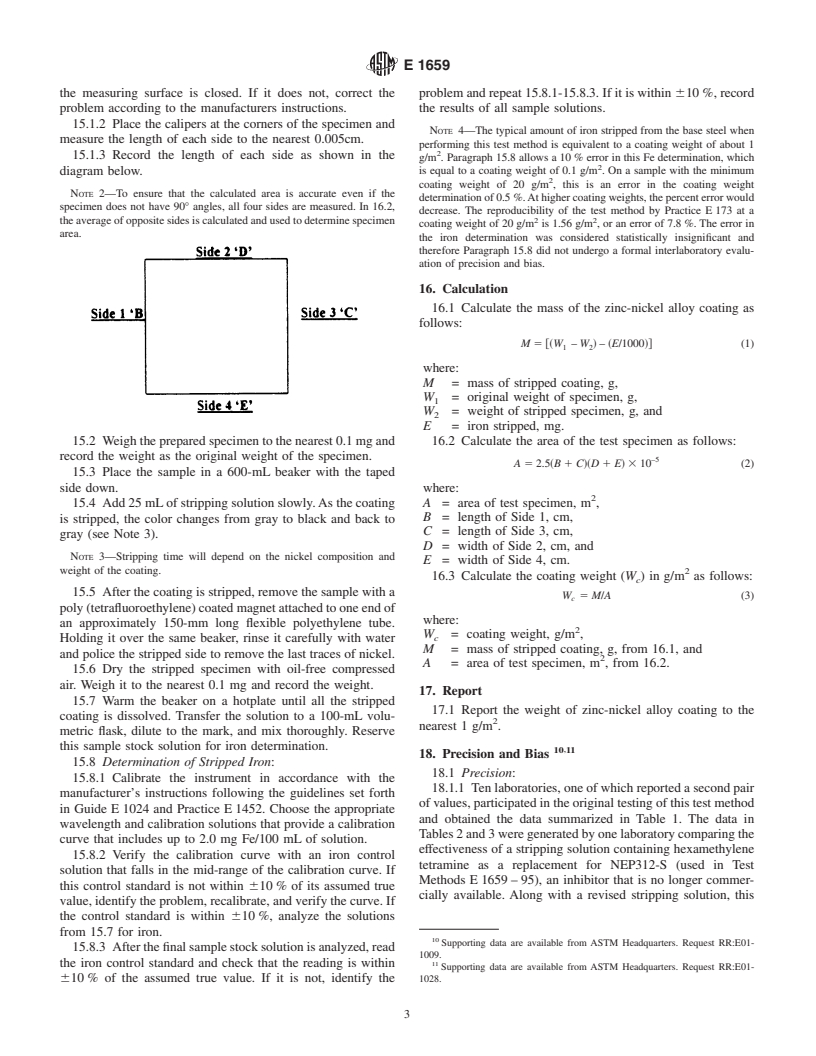
15.1 Specimen Area—Using the calipers, measure and
listed by the American Chemical Society, see Analar Standards for Laboratory
record the length of all four sides of the test specimen.
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
15.1.1 Check that the measuring face and reference edge of
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
MD. the calipers are clean. Check that the calipers read “0” when
E1659
the measuring surface is closed. If it does not, correct the problem and repeat 15.8.1-15.8.3. If it is within 610 %, record
problem according to the manufacturers instructions. the results of all sample solutions.
15.1.2 Place the calipers at the corners of the specimen and
NOTE 4—The typical amount of iron stripped from the base steel when
measure the length of each side to the nearest 0.005cm.
performing this test method is equivalent to a coating weight of about 1
15.1.3 Record the length of each side as shown in the
g/m . Paragraph 15.8 allows a 10 % error in this Fe determination, which
diagram below. is equal to a coating weight of 0.1 g/m . On a sample with the minimum
coating weight of 20 g/m , this is an error in the coating weight
NOTE 2—To ensure that the calculated area is accurate even if the
determinationof0.5 %.Athighercoatingweights,thepercenterrorwould
specimen does not have 90° angles, all four sides are measured. In 16.2,
decrease. The reproducibility of the test method by Practice E 173 at a
theaverageofoppositesidesiscalculatedandusedtodeterminespecimen 2 2
coating weight of 20 g/m is 1.56 g/m , or an error of 7.8 %. The error in
area.
the iron determination was considered statistically insignificant and
therefore Paragraph 15.8 did not undergo a formal interlaboratory evalu-
ation of precision and bias.
16. Calculation
16.1 Calculate the mass of the zinc-nickel alloy coating as
follows:
M 5 @~W – W !– ~E/1000!# (1)
1 2
where:
M = mass of stripped coating, g,
W = original weight of specimen, g,
W = weight of stripped specimen, g, and
E = iron stripped, mg.
15.2 Weighthepreparedspecimentothenearest0.1mgand 16.2 Calculate the area of the test specimen as follows:
record the weight as the original weight of the specimen.
–5
A 5 2.5~B 1 C!~D 1 E! 3 10 (2)
15.3 Place the sample in a 600-mL beaker with the taped
side down.
where:
15.4 Add 25 mLof stripping solution slowly.As the coating A = area of test specimen, m ,
B = length of Side 1, cm,
is stripped, the color changes from gray to black and back to
C = length of Side 3, cm,
gray (see Note 3).
D = width of Side 2, cm, and
NOTE 3—Stripping time will depend on the nickel composition and
E = width of Side 4, cm.
weight of the coating. 2
16.3 Calculate the coating weight (W)ing/m as follows:
c
15.5 After the coating is stripped, remove the sample with a
W 5 M/A (3)
c
poly(tetrafluoroethylene)coatedmagnetattachedtooneendof
where:
an approximately 150-mm long flexible polyethylene tube.
W = coating weight, g/m ,
Holding it over the same beaker, rinse it carefully with water c
M = mass of stripped coating, g, from 16.1, and
and police the stripped side to remove the last traces of nickel.
A = area of test specimen, m , from 16.2.
15.6 Dry the stripped specimen with oil-free compressed
air. Weigh it to the nearest 0.1 mg and record the weight.
17. Report
15.7 Warm the beaker on a hotplate until all the stripped
17.1 Report the weight of zinc-nickel alloy coating to the
coating is dissolved. Transfer the solution to a 100-mL volu-
nearest 1 g/m .
metric flask, dilute to the mark, and mix thoroughly. Reserve
this sample stock solution for iron determination.
,
10 11
18. Precision and Bias
15.8 Determination of Stripped Iron:
18.1 Precision:
15.8.1 Calibrate the instrument in accordance with the
18.1.1 Ten laboratories, one of which reported a second pair
manufacturer’s instructions following the guidelines set forth
of values, participated in the original testing of this test method
in Guide E 1024 and Practice E 1452. Choose the appropriate
and obtained the data summarized in Table 1. The data in
wavelength and calibration solutions that provide a calibration
Tables2and3weregeneratedbyonelaboratorycomparingthe
curve that includes up to 2.0 mg Fe/100 mL of solution.
effectiveness of a stripping solution containing hexamethylene
15.8.2 Verify the calibration curve with an iron control
tetramine as a replacement for NEP312-S (used in Test
solution that falls in the mid-range of the calibration curve. If
Methods E 1659 – 95), an inhibitor that is no longer commer-
this control standard is not within 610 % of its assumed true
cially available. Along with a revised stripping solution, this
value, identify the problem, recalibrate, and verify the curve. If
the control standard is within 610 %, analyze the solutions
from 15.7 for iron.
Supporting data are available from ASTM Headquarters. Request RR:E01-
15.8.3 Afterthefinalsamplestocksolutionisanalyzed,read
1009.
the iron control standard and check that the reading is within
Supporting data are available from ASTM Headquarters. Request RR:E01-
610 % of the assumed true value. If it is not, identify the 1028.
E1659
TABLE 1 Statistical Information—Coating Weight-Original Test
due to iron being stripped from the base metal. This bias is
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.