ASTM D5357-03(2008)e1
(Test Method)Standard Test Method for Determination of Relative Crystallinity of Zeolite Sodium A by X-ray Diffraction
Standard Test Method for Determination of Relative Crystallinity of Zeolite Sodium A by X-ray Diffraction
SIGNIFICANCE AND USE
Zeolite NaA has been used as an active component in molecular sieves employed as desiccants for natural gas, process gas streams, sealed insulated windows, and as a builder (water softener) in household laundry detergents.
This X-ray procedure is designed to allow a reporting of the relative degree of crystallization of NaA in the manufacture of NaA. The relative crystallinity number has proven useful in technology, research, and specifications.
Drastic changes in intensity of individual peaks in the XRD pattern of NaA can result from changes in distribution of electron density within the unit cell of the NaA zeolite. The electron density distribution is dependent upon the extent of filling of pores in the zeolite with guest molecules, and on the nature of the guest molecules. In this XRD method, the guest molecule H2O completely fills the pores. Intensity changes may also result if some or all of the sodium cations in NaA are exchanged by other cations.
Drastic changes in overall intensity can result from changes in X-ray absorption attributed to non-crystalline phases, if present, in a NaA sample. If non-zeolite crystalline phases are present, their diffraction peaks may overlap with some of the NaA diffraction peaks selected for this test method. If there is reason to suspect the presence of such components, then NaA peaks free of interference should be chosen for analysis.
SCOPE
1.1 This test method covers a procedure for determining the relative crystallinity of zeolite sodium A (zeolite NaA) using selected peaks from the X-ray diffraction pattern of the zeolite.
1.2 The term “intensity of an X-ray powder diffraction (XRD) peak” refers to the “integral intensity,” either the area or counts under the peak or the product of the peak height and the peak width at half height.
1.3 This test method provides a number that is the ratio of intensity of portions of the XRD pattern of the sample to intensity of the corresponding portion of the pattern of a reference zeolite NaA. The intensity ratio, expressed as a percentage, is then labeled relative crystallinity of NaA.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: D5357 − 03(Reapproved 2008)
Standard Test Method for
Determination of Relative Crystallinity of Zeolite Sodium A
by X-ray Diffraction
This standard is issued under the fixed designation D5357; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—The units statement in subsection 1.4 was corrected editorially in May 2008.
1. Scope E691 Practice for Conducting an Interlaboratory Study to
Determine the Precision of a Test Method
1.1 This test method covers a procedure for determining the
relative crystallinity of zeolite sodium A (zeolite NaA) using
3. Summary of Test Method
selected peaks from the X-ray diffraction pattern of the zeolite.
3.1 The XRD patterns of the zeolite NaA or zeolite NaA-
1.2 The term “intensity of an X-ray powder diffraction
(XRD)peak”referstothe“integralintensity,”eithertheareaor containing sample and the reference sample (NaA) are ob-
tained under the same conditions.Acomparison of the sums of
counts under the peak or the product of the peak height and the
peak width at half height. intensities of six strong peaks in the 11–32° 2θ range is made,
giving relative crystallinity of NaA.This type of comparison is
1.3 This test method provides a number that is the ratio of
commonlyusedinzeolitetechnologyandisoftenreferredtoas
intensity of portions of the XRD pattern of the sample to
“% crystallinity.”
intensity of the corresponding portion of the pattern of a
reference zeolite NaA. The intensity ratio, expressed as a
4. Significance and Use
percentage, is then labeled relative crystallinity of NaA.
4.1 Zeolite NaA has been used as an active component in
1.4 The values stated in SI units are to be regarded as
molecular sieves employed as desiccants for natural gas,
standard. No other units of measurement are included in this
standard. processgasstreams,sealedinsulatedwindows,andasabuilder
(water softener) in household laundry detergents.
1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
4.2 This X-ray procedure is designed to allow a reporting of
responsibility of the user of this standard to establish appro-
the relative degree of crystallization of NaAin the manufacture
priate safety and health practices and determine the applica-
of NaA. The relative crystallinity number has proven useful in
bility of regulatory limitations prior to use.
technology, research, and specifications.
2. Referenced Documents 4.3 Drastic changes in intensity of individual peaks in the
XRD pattern of NaAcan result from changes in distribution of
2.1 ASTM Standards:
electron density within the unit cell of the NaA zeolite. The
D3906 Test Method for Determination of Relative X-ray
electron density distribution is dependent upon the extent of
Diffraction Intensities of Faujasite-Type Zeolite-
filling of pores in the zeolite with guest molecules, and on the
Containing Materials
nature of the guest molecules. In this XRD method, the guest
E177 Practice for Use of the Terms Precision and Bias in
molecule H O completely fills the pores. Intensity changes
ASTM Test Methods
may also result if some or all of the sodium cations in NaAare
E456 Terminology Relating to Quality and Statistics
exchanged by other cations.
4.4 Drastic changes in overall intensity can result from
This test method is under the jurisdiction of ASTM Committee D32 on
changes in X-ray absorption attributed to non-crystalline
Catalysts and is the direct responsibility of Subcommittee D32.05 on Zeolites.
phases, if present, in a NaA sample. If non-zeolite crystalline
Current edition approved April 16, 2008. Published May 2008. Originally
phases are present, their diffraction peaks may overlap with
approved in 1993. Last previous edition approved in 2003 as D5357-03. DOI:
10.1520/D5357-03R08E01.
someoftheNaAdiffractionpeaksselectedforthistestmethod.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
If there is reason to suspect the presence of such components,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
then NaA peaks free of interference should be chosen for
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. analysis.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
D5357 − 03 (2008)
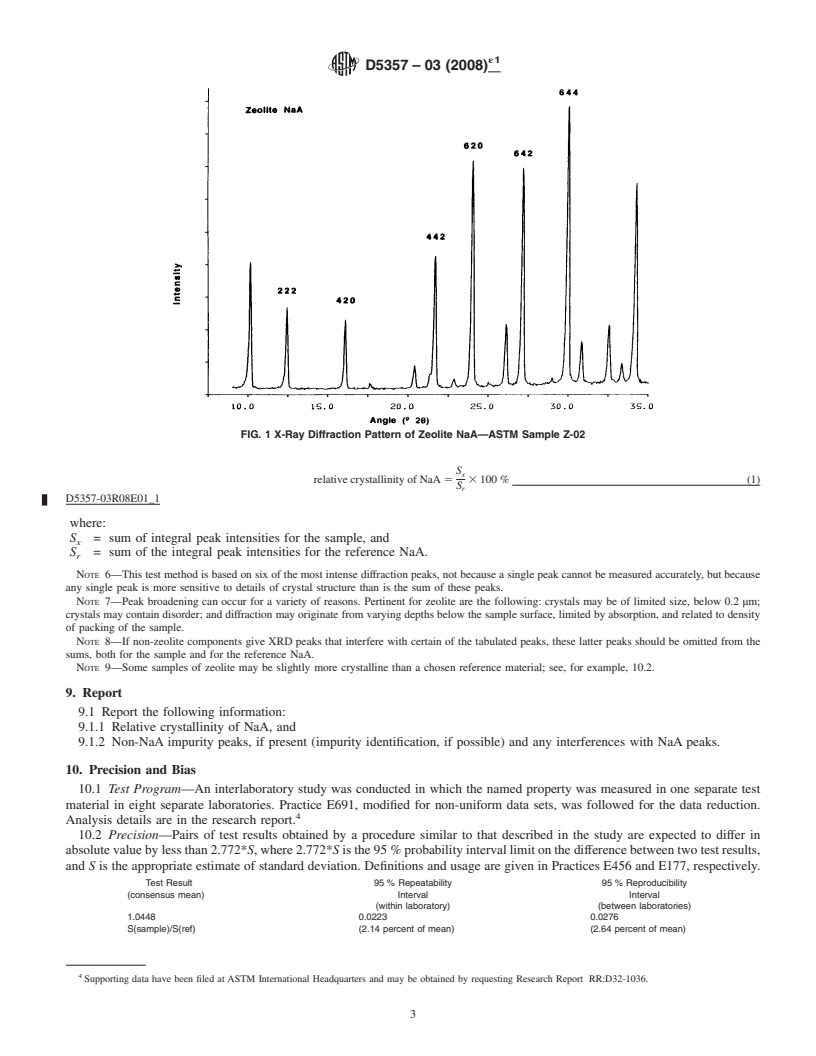
FIG. 1 X-Ray Diffraction Pattern of Zeolite NaA—ASTM Sample Z-02
5. Apparatus 7.1.2 Pack the sample into an XRD sample holder.
7.1.3 Obtain an XRD pattern of the NaA reference by
5.1 X–ray Diffractometer, equipped with computerized data
scanning over the angle range from 11 to 32° 2θ at 0.25°/min.
acquisition and reduction capability or with a strip chart
In the step mode, a 0.02° 2θ step for 2 s may be acceptable for
recorder, and using copper K-alpha radiation.
pure NaA, while 10 to 20 s may be necessary for lower NaA
5.2 Drying Oven, set at 100°C.
content samples. This scan range includes the six strong
5.3 Hydrator (Laboratory Desiccator), maintained at about diffraction peaks that are to be used in the calculation for “%
crystallinity”:
58 % relative humidity by a saturated solution of sodium
bromide, NaBr.
hkl index d (Angstrom) °2θ (Cu K-α radiation)
222 7.104 12.46
5.4 Planimeter or Appropriate Peak Profile Analysis or
420 5.503 16.11
Digital Integration Software, if diffractometer is not equipped
442 4.102 21.67
620 3.710 23.99
with appropriate software data analysis capability.
642 3.289 27.12
644 2.984 29.94
6. Reagents and Materials
Fig. 1 shows a pattern for the reference zeolite NaA used in
6.1 NaA Powder , as reference standard, preferably with a
testing of this method.
mean particle diameter of 3 to 5 microns (mean crystal size 1
NOTE 3—1 nanometer (nm) = 10 Angstroms.
to 2 microns).
7.1.3.1 If a strip chart recorde
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation:D5357–03 (Reapproved 2008)
Standard Test Method for
Determination of Relative Crystallinity of Zeolite Sodium A
by X-ray Diffraction
This standard is issued under the fixed designation D5357; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—The units statement in subsection 1.4 was corrected editorially in May 2008.
1. Scope
1.1 This test method covers a procedure for determining the relative crystallinity of zeolite sodium A (zeolite NaA) using
selected peaks from the X-ray diffraction pattern of the zeolite.
1.2 The term “intensity of an X-ray powder diffraction (XRD) peak” refers to the “integral intensity,” either the area or counts
under the peak or the product of the peak height and the peak width at half height.
1.3 This test method provides a number that is the ratio of intensity of portions of the XRD pattern of the sample to intensity
ofthecorrespondingportionofthepatternofareferencezeoliteNaA.Theintensityratio,expressedasapercentage,isthenlabeled
relative crystallinity of NaA.
1.4
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D3906 Test Method for Determination of Relative X-ray Diffraction Intensities of Faujasite-Type Zeolite-Containing Materials
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E456 Terminology Relating to Quality and Statistics
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3. Summary of Test Method
3.1 The XRD patterns of the zeolite NaAor zeolite NaA-containing sample and the reference sample (NaA) are obtained under
the same conditions. A comparison of the sums of intensities of six strong peaks in the 11–32° 2u range is made, giving relative
crystallinity of NaA. This type of comparison is commonly used in zeolite technology and is often referred to as “% crystallinity.”
4. Significance and Use
4.1 Zeolite NaAhas been used as an active component in molecular sieves employed as desiccants for natural gas, process gas
streams, sealed insulated windows, and as a builder (water softener) in household laundry detergents.
4.2 This X-ray procedure is designed to allow a reporting of the relative degree of crystallization of NaA in the manufacture
of NaA. The relative crystallinity number has proven useful in technology, research, and specifications.
4.3 Drastic changes in intensity of individual peaks in the XRD pattern of NaA can result from changes in distribution of
electron density within the unit cell of the NaA zeolite. The electron density distribution is dependent upon the extent of filling
of pores in the zeolite with guest molecules, and on the nature of the guest molecules. In this XRD method, the guest molecule
H O completely fills the pores. Intensity changes may also result if some or all of the sodium cations in NaA are exchanged by
other cations.
4.4 Drastic changes in overall intensity can result from changes in X-ray absorption attributed to non-crystalline phases, if
present, in a NaAsample. If non-zeolite crystalline phases are present, their diffraction peaks may overlap with some of the NaA
This test method is under the jurisdiction of ASTM Committee D32 on Catalysts and is the direct responsibility of Subcommittee D32.05 on Zeolites.
Current edition approved April 1, 2008. Published May 2008. Originally approved in 1993. Last previous edition approved in 2003 as D5357-03. DOI:
10.1520/D5357-03R08E01.
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
D5357–03 (2008)
diffraction peaks selected for this test method. If there is reason to suspect the presence of such components, then NaApeaks free
of interference should be chosen for analysis.
5. Apparatus
5.1 X–ray Diffractometer, equipped with computerized data acquisition and reduction capability or with a strip chart recorder,
and using copper K-alpha radiation.
5.2 Drying Oven, set at 100°C.
5.3 Hydrator (Laboratory Desiccator), maintained at about 58 % relative humidity by a saturated solution of sodium bromide,
NaBr.
5.4 Planimeter or Appropriate Peak Profile Analysis or Digital Integration Software, if diffractometer is not equipped with
appropriate software data analysis capability.
6. Reagents and Materials
6.1 NaA Powder , as reference standard, preferably with a mean particle diameter of 3 to 5 microns (mean crystal size 1 to 2
microns).
7. Procedure
7.1 Carry out the steps (described in 7.1.1-7.1.3) in an identical manner for both the sample and the NaA reference.
7.1.1 Place about 1.5 g of finely divided sample in the drying oven at 100°C for 2 h. Cool the sample in the hydrator and hold
there at room temperature and about 58 % relative humidity for at least 16 h.
NOTE 1—Grinding of course-textured samples should be done gently. Over-grinding can lead to breaking up of fine crystals and destruction of the
zeolite.
NOTE 2—Drying followed by rehydration results in filling the zeolite pores with water of hydration but without an excess of moisture residing on the
surface of the zeolite particles.
7.1.2 Pack the sample into an XRD samp
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.