ASTM F2638-22
(Test Method)Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
SIGNIFICANCE AND USE
6.1 This test method has been developed as a result of research performed by Air Dispersion Limited (Manchester, UK) and funded by the Barrier Test Consortium Limited. The results of this research have been published in a peer-reviewed journal.4 This research demonstrated that testing the barrier performance of porous packaging materials using microorganisms correlates with measuring the filtration efficiency of the materials.
6.2 This test method does not require the use of microbiological method; in addition, the test method can be conducted in a rapid and timely manner.
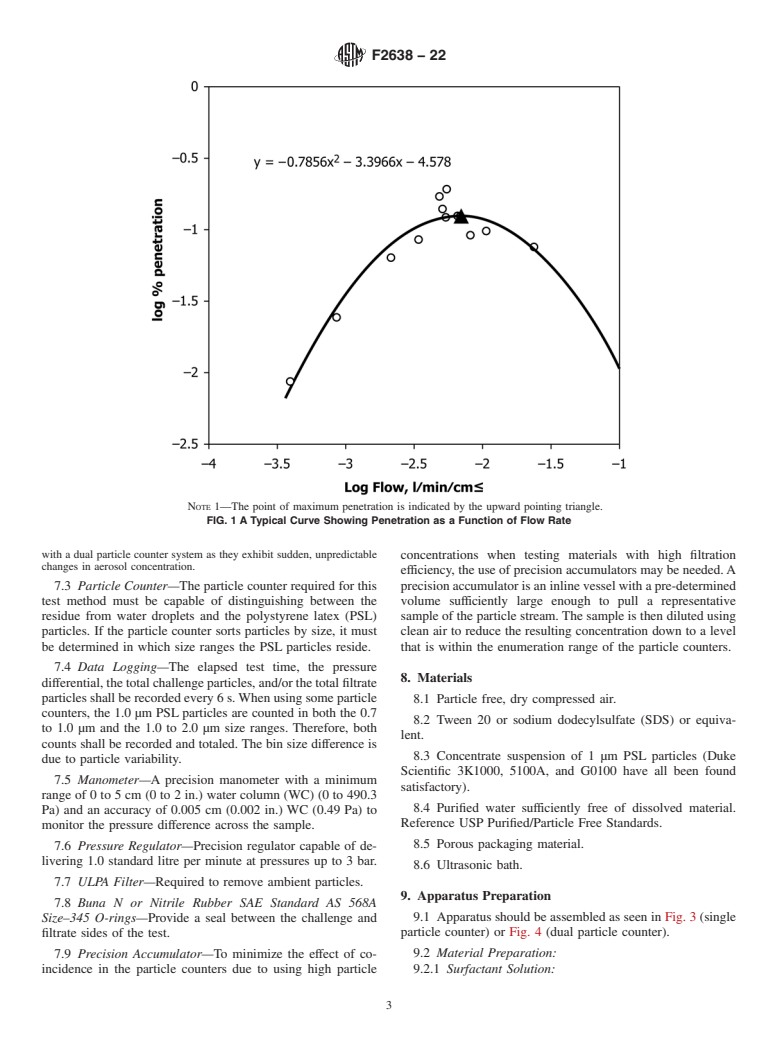
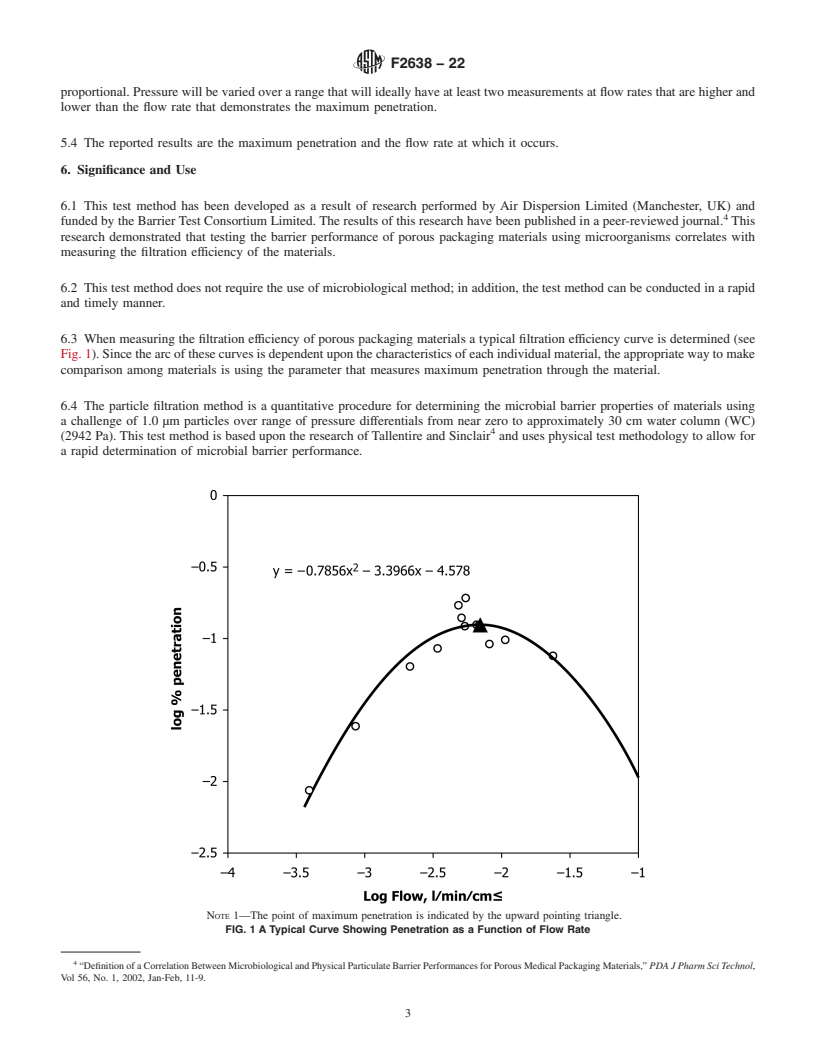
6.3 When measuring the filtration efficiency of porous packaging materials a typical filtration efficiency curve is determined (see Fig. 1). Since the arc of these curves is dependent upon the characteristics of each individual material, the appropriate way to make comparison among materials is using the parameter that measures maximum penetration through the material.
FIG. 1 A Typical Curve Showing Penetration as a Function of Flow Rate
Note 1: The point of maximum penetration is indicated by the upward pointing triangle.
6.4 The particle filtration method is a quantitative procedure for determining the microbial barrier properties of materials using a challenge of 1.0 µm particles over range of pressure differentials from near zero to approximately 30 cm water column (WC) (2942 Pa). This test method is based upon the research of Tallentire and Sinclair4 and uses physical test methodology to allow for a rapid determination of microbial barrier performance.
SCOPE
1.1 This test method measures the aerosol filtration performance of porous packaging materials by creating a defined aerosol of 1.0 μm particles and assessing the filtration efficiency of the material using either single or dual particle counters.
1.2 This test method is applicable to porous materials used to package terminally sterilized medical devices.
1.3 The intent of this test apparatus is to determine the flow rate through a material at which maximum penetration occurs.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2638 − 22
Standard Test Method for
Using Aerosol Filtration for Measuring the Performance of
Porous Packaging Materials as a Surrogate Microbial
1
Barrier
This standard is issued under the fixed designation F2638; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to
1.1 This test method measures the aerosol filtration perfor-
Determine the Precision of a Test Method
mance of porous packaging materials by creating a defined
3
2.2 ISO Standard:
aerosol of 1.0 µm particles and assessing the filtration effi-
ISO 5636–3 Paper and Board—Determination of Air Per-
ciency of the material using either single or dual particle
meance (Medium Range)—Part 3: Bendtsen Method
counters.
1.2 This test method is applicable to porous materials used
3. Terminology
to package terminally sterilized medical devices.
3.1 Definitions:
1.3 The intent of this test apparatus is to determine the flow
3.1.1 challenge aerosol—a sufficient quantity of aerosolized
rate through a material at which maximum penetration occurs.
1.0 µm particles that enable effective particle counting in the
1.4 The values stated in SI units are to be regarded as the
filtrate aerosol.
standard. The values given in parentheses are for information
3.1.2 filtrate aerosol—particlesthatremainaerosolizedafter
only.
passage through the test specimen.
1.5 This standard does not purport to address all of the
3.1.3 maximum penetration—the highest percent concentra-
safety concerns, if any, associated with its use. It is the
tionofparticlesinthefiltrateaerosolwhenaspecimenistested
responsibility of the user of this standard to establish appro-
over a range of pressure differentials or air flow rates.
priate safety, health, and environmental practices and deter-
3.2 Abbreviations and Symbols:
mine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accor- Symbol Unit Description
C n Average particle count of the challenge aerosol
S
dance with internationally recognized principles on standard-
when using a single particle counter (Method A).
ization established in the Decision on Principles for the
C n Average particle count of the filtrate aerosol.
F
C n Average particle count of the challenge aerosol.
Development of International Standards, Guides and Recom- C
C N Average particle count of the filtrate aerosol prior to
LR
mendations issued by the World Trade Organization Technical
correction for dilution.
Barriers to Trade (TBT) Committee.
R % Percentage of particles from the challenge aerosol
that remain in the filtrate aerosol.
R % The calculated maximum of R.
M
2. Referenced Documents
P cm WC Pressure differential across a test specimen due to
1
2
2.1 ASTM Standards: the air flow required by the particle counter.
P cm WC Pressure differential across a test specimen.
E171/E171M Practice for Conditioning and Testing Flexible
2
F L/m/cm Air flow rate through the test specimen.
Barrier Packaging 2
F L/m/cm Air flow rate required by the particle counter when
1
E177 Practice for Use of the Terms Precision and Bias in measuring the filtrate aerosol.
2
F L/m/cm Air flow rate at which maximum penetration occurs.
M
1 4. Safety
This test method is under the jurisdiction ofASTM Committee F02 on Primary
Barrier Packaging and is the direct responsibility of Subcommittee F02.15 on
4.1 The waste and the vacuum venturi vents for the test
Chemical/Safety Properties.
equipment described in this test method emit an aerosol of
Current edition approved May 1, 2022. Published June 2022. Originally
approved in 2007. Last previous edition approved in 2018 as F2638 – 18. DOI: polystyrene particles and salt residues. These aerosols should
10.1520/F2638-22.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2638 − 22
4
be exhausted from any enclosed environment or collected and journal. This research demonstrated
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2638 − 18 F2638 − 22
Standard Test Method for
Using Aerosol Filtration for Measuring the Performance of
Porous Packaging Materials as a Surrogate Microbial
1
Barrier
This standard is issued under the fixed designation F2638; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method measures the aerosol filtration performance of porous packaging materials by creating a defined aerosol of
1.0 μm particles and assessing the filtration efficiency of the material using either single or dual particle counters.
1.2 This test method is applicable to porous materials used to package terminally sterilized medical devices.
1.3 The intent of this test apparatus is to determine the flow rate through a material at which maximum penetration occurs.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E171/E171M Practice for Conditioning and Testing Flexible Barrier Packaging
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3
2.2 ISO Standard:
ISO 5636–3 Paper and Board—Determination of Air Permeance (Medium Range)—Part 3: Bendtsen Method
3. Terminology
3.1 Definitions:
1
This test method is under the jurisdiction of ASTM Committee F02 on Primary Barrier Packaging and is the direct responsibility of Subcommittee F02.15 on
Chemical/Safety Properties.
Current edition approved Sept. 1, 2018May 1, 2022. Published September 2018June 2022. Originally approved in 2007. Last previous edition approved in 20122018 as
ɛ1
F2638 – 12F2638 – 18. . DOI: 10.1520/F2638-18.10.1520/F2638-22.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2638 − 22
3.1.1 challenge aerosol—a sufficient quantity of aerosolized 1.0 μm particles that enable effective particle counting in the filtrate
aerosol.
3.1.2 filtrate aerosol—particles that remain aerosolized after passage through the test specimen.
3.1.3 maximum penetration—the highest percent concentration of particles in the filtrate aerosol when a specimen is tested over
a range of pressure differentials or air flow rates.
3.2 Abbreviations and Symbols:
Symbol Unit Description
C n Average particle count of the challenge aerosol
S
when using a single particle counter (Method A).
C n Average particle count of the filtrate aerosol.
F
C n Average particle count of the challenge aerosol.
C
C N Average particle count of the filtrate aerosol prior to
LR
correction for dilution.
R % Percentage of particles from the challenge aerosol
that remain in the filtrate aerosol.
R % The calculated maximum of R.
M
P cm WC Pressure differential across a test specimen due to
1
the air flow required by the particle counter.
P cm WC Pressure differential across a test specimen.
2
F L/m/cm Air flow rate through the test specimen.
2
F L/m/cm Air flow rate required by the particle counter when
1
measuring the filtrate aerosol.
2
F L/m/cm Air flow rate at which maximum penetration occurs.
M
4. Safety
4.1 The waste and the vacuum venturi vents for the test equipment described in th
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.