ASTM D5492-98
(Test Method)Standard Test Method for Determination of Xylene Solubles in Propylene Plastics
Standard Test Method for Determination of Xylene Solubles in Propylene Plastics
SCOPE
1.1 This test method is to be used for determining the 25°C ortho-xylene-soluble fraction of polypropylene and propylene-ethylene copolymers.
1.2 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Note 1-This standard is similar to ISO 6427-1982 in title only. The technical content is significantly different.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 5492 – 98
Standard Test Method for
Determination of Xylene Solubles in Propylene Plastics
This standard is issued under the fixed designation D 5492; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope %. When the solution is cooled the insoluble portion precipi-
tates and is isolated by filtration. The orthoxylene is evaporated
1.1 This test method is to be used for determining the 25°C
from the filtrate, leaving the soluble fraction in the residue. The
ortho-xylene-soluble fraction of polypropylene and propylene-
percentage of this fraction in the plastic is determined gravi-
ethylene copolymers.
metrically.
1.2 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
5. Significance and Use
responsibility of the user of this standard to establish appro-
5.1 The results of this test provide a relative measure of the
priate safety and health practices and determine the applica-
total soluble fraction of polypropylene and copolymers. The
bility of regulatory limitations prior to use.
soluble fraction can be approximately correlated to the amor-
NOTE 1—This standard is similar to ISO 6427-1982 in title only. The
phous fraction in the polypropylene. Xylene is widely used for
technical content is significantly different.
determining the soluble fraction in polypropylene. The concen-
tration of a soluble fraction obtained with a specific solvent has
2. Referenced Documents
been found to relate closely to the performance characteristics
2.1 ASTM Standards:
of a product in certain applications, for example film and fiber.
D 883 Terminology Relating to Plastics
Data obtained by one solvent and at one precipitation time
D 1600 Terminology of Abbreviated Terms Relating to
cannot be compared with data obtained by another solvent or
Plastics
precipitation time, respectively. Xylene is more specific to the
2.2 ISO Standard:
atactic fraction than other solvents.
ISO 6427-1982 Plastics—Determination of Matter Ex-
tracted by Organic Solvents (Conventional Methods) An-
6. Interferences
nex B Standard Method of Test for Determination of
6.1 Materials with solubilities similar to the polymer frac-
Polypropylene Solubility in Cold Xylene
tion, such as additives, may interfere with the measurement of
solubles. When present in concentrations that are judged to
3. Terminology
impart a significant error to the soluble-fraction data, the level
3.1 For definitions of plastic terms see Terminology D 883
of interference must be determined and corrections made.
and for abbreviations see Terminology D 1600.
6.2 Small-particle fillers and pigments that may pass
3.2 There are no terms in this test method that require new
through the filter and insoluble gels present in the polymer may
or other-than-dictionary definitions.
cause errors in the measurement.
6.3 The polymer flakes and spheres must be dried before
4. Summary of Test Method
testing to eliminate moisture that can influence the initial
4.1 A weighed amount of sample is dissolved in orthoxylene
weight of sample added to the flask.
under reflux conditions. The solution is cooled under con-
trolled conditions and maintained at a 25°C equilibrium
7. Apparatus
temperature so that the crystallization of the insoluble fraction
7.1 Reflux-Condenser Apparatus, 400 mm, with 24/40 glass
takes place. One of two precipitation-time periods can be used,
joint.
although the longer precipitation time should be used for
7.2 Flat-Bottom Boiling Flask, with one or two necks, 400
homopolymers and copolymers with solubles less than 12 mass
mL with 24/40 joint, Erlenmeyer flask, or cylindrical bottle.
7.3 Insulation Disk, made of fiberglass or rock wool.
7.4 Electromagnetic Stirrer, with temperature-controlled
This test method is under the jurisdiction of ASTM Committee D-20 on Plastics
and is the direct responsibility of Subcommittee D20.12 on Olefin Plastics.
heating plate, thermostated oil bath, or heater block capable of
Current edition approved Aug. 10, 1998. Published February 1999. Originally
maintaining 145 to 150°C.
published as D 5492 – 94.
2 7.5 Stirring Bar.
Annual Book of ASTM Standards, Vol 08.01.
7.6 Pipette, Class A, 200 mL.
Available from American National Standards Institute, 11 W. 42nd St., 13th
Floor, New York, 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5492
7.7 Pipette, Class A, 100 mL. flask. Continue the filtration until all the filtrate has been
7.8 Glass-Stoppered Volumetric Flask, 250 mL. collected.
7.9 Thermostatically Controlled Water Bath, at 25°C. 9.2.5 Dry the aluminum pans for 30 min in an oven at
7.10 Electromagnetic Stirrers. 150°C. Cool the pans in a desiccator until ready to use. For
7.11 Filter Paper, fluted, Whatman No. 4, No. 541, or each sample weigh a clean, dry pan on the analytical balance to
equivalent, 200 mm. the nearest 0.0001 g.
7.12 Funnel, 60°, 200 mm. 9.2.6 With a Class A pipette, pipet a 100-mL aliquot of the
7.13 Heated Vacuum Oven. filtered o-xylene into the weighed aluminum pan.
7.14 Disposable Aluminum Pans, 300-mL capacity, with 9.2.7 Place the pan on a temperature-controlled heating
smooth sides. plate maintained at 145 to 150°C. Allow the aliquot to obtain
7.15 Temperature-Controlled Heating Plate. a rolling boil to prevent splashing. Blanket the pan with a slow
7.16 Analytical Balance, with minimum weighing sensitiv- stream of nitrogen. Continue heating the pan until the residue
ity to 0.0001 g (a sensitivity of 0.00001 is preferred). in the dish is almost dry.
7.17 Desiccator, containing appropriate desiccant. 9.2.8 Place the pan into a vacuum oven at 100 6 5°C for
7.18 Timer with Alarms, in minutes. about an hour at a pressure less than 13.3 kPa.
9.2.9 Cool the pan to room temperature in a desiccator and
8. Reagents
weigh the pan to the nearest 0.0001 g. Repeat the drying,
8.1 Reagent-Grade Ortho-Xylene (o-Xylene)—Assay gas
cooling, and weighing steps until two consecutive weighings
chromatography (GC) = 98 % min; less than 2 % ethylbenzene
agree within 0.0002 g. Calculate the average blank-residual
as established by GC; evaporation residue at 140°C less than
mass of the three determinations.
0.002 g/100 mL; boiling point 144°C.
9.3 Determine the Percent Soluble Fraction in the Polymer:
9.3.1 Dry the polypropylene powder or spheres before
9. Procedure
analysis. It is not necessary to dry the pellets unless it is known
9.1 Preparation of the o-Xylene:
that they contain high levels of moisture pellets or spheres
9.1.1 It is not necessary to stabilize the o-xylene before
before analysis. If necessary, dry the samples in a vacuum oven
using, but it may be stabilized if desired.
at 70 6 5°C, in a vacuum of 13.3 kPa for a minimum of 20
5 6 7
NOTE 2—BHT, Irganox 1010, and Santonox R have been found to be
min. Cool the sample in a desiccator to prevent moisture
effective stabilizers for o-xylene.
pickup.
9.1.2 Degas the o-xylene. Using nitrogen gas, purge the
NOTE 4—For large pellets or spheres, where there is concern that the
o-xylene for a minimum of 1 h every 24 h.
polymer sample will not dissolve in a reasonable time frame, the pellets or
9.2 Determine the Level of Contamination in the o-Xylene
spheres may be ground to an appropriate size to afford a faster dissolution.
(Solvent Blank): Ground material should be dried as specified in 9.3.1.
9.2.1 The purpose of the solvent blank is to determine
9.3.2 Weigh out a sample in accordance with Table 1. When
whether the o-xylene to be used contains significant amounts of
the expected solubles level is unknown use a 2.0 6 0.1 g
evaporation residue or foreign components. A solvent-blank
sample. For referee testing between laboratories a sample
test for residue should be run on every new lot of o-xylene.
2.0 6 0.1 g shall be used, unless there is agreement between
Run and average the solvent-blank results, for three aliquots
the laboratories to use a different sample size. Determine mass
per bottle or lot of o-xylene. Each aliquot shall be 200 mL.
of the sample to the nearest 0.0001 g. Pour the sample into a
flat-bottom boiling flask. Place a magnetic stirring bar in the
NOTE 3—It is recommended that o-xylene be purchased in glass or
flask.
glass-lined containers and of a size such that the o-xylene will be used
within three days, once opened. Containers of larger size may be used if
NOTE 5—Table 1 provides a choice of sample mass. Use the largest
the o-xylene is used up within a short period of time. The purpose of the
sample mass possible to minimize variability of the test data, unless from
short time period is to ensure purity and minimize moisture pickup and
prior experience it is known that the polymer/o-xylene solution does not
other contaminants.
filter readily as in 9.3.11.
9.2.2 Pipet 200 mL of unstabilized or stabilized o-xylene
9.3.3 Pipet 200 mL of unstabilized or stabilized o-xylene
into a clean empty flask.
into the flask.
9.2.3 Place a 200-mm No. 4 filter paper or equivalent in a
9.3.4 Attach the flask to the condenser.
200-mm funnel in a funnel rack over a 250-mL glass-stoppered
9.3.5 Place an insulation disk on top of the electromagnetic
flask.
stirrer plate to prevent localized heating of the flask. Position
9.2.4 For each sample blank, pour the contents from the
the flask and condenser system on top of the insulation disk
flask into a funnel and allow the filtrate to drip into a second
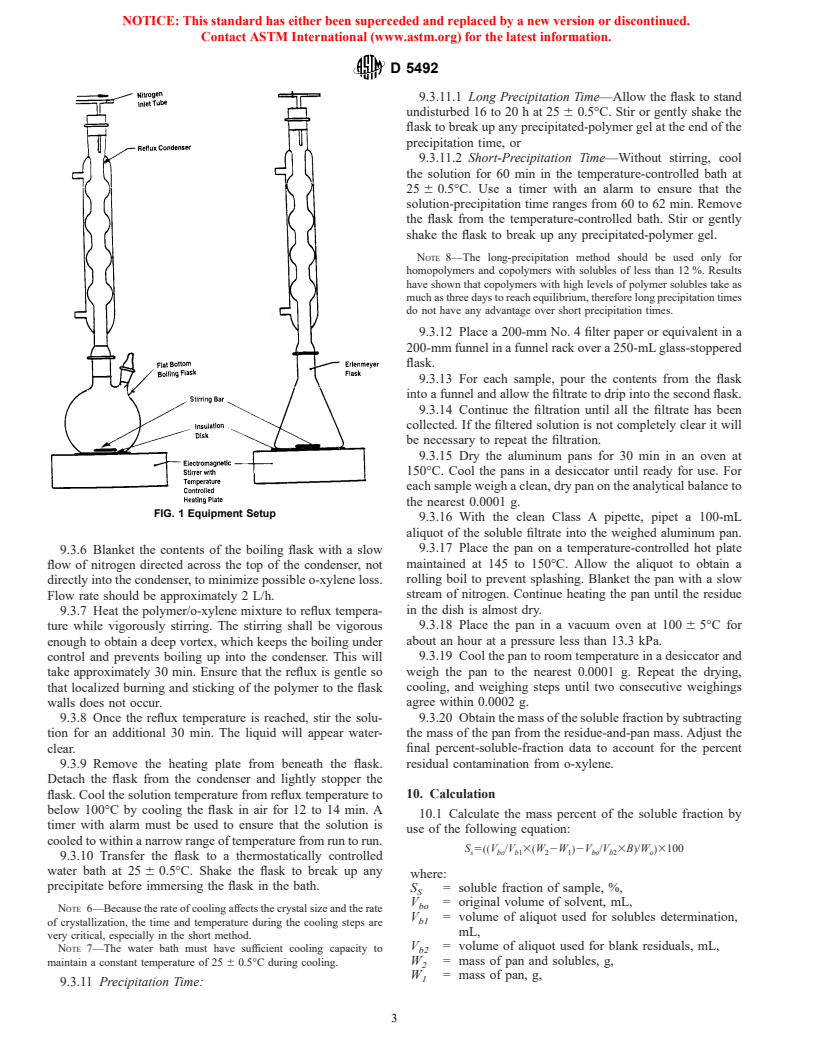
(see Fig. 1). Position the nitrogen inlet tube in the top of the
condenser. Turn on the cooling water to the condenser.
Available from Whatman Intn. Ltd., Maidstone, England or from Fisher
TABLE 1 Sample Size
Scientific, 711 Forbes Ave., Pittsburgh, PA 15219.
A
Available from Uniroyal Chemical Co., Inc., Specialty Chemicals Division,
Expected Solubles Initial Sample Mass, g
World Headquarters, Benson Rd., Middlebury, CT 06749.
6 <8 % by mass 4.0000 6 0.1000 or 2.000 6 0.1000
Available from CIBA-GEIGY Corp., Additive Division, Seven Skyline Drive,
8.0 to 30.0 % by mass 2.0000 6 0.1000
Hawthorne, NY 10532.
>30.0 % by mass 2.0000 6 0.1000 or 1.0000 6 0.1000
Available from Monsanto Co., Chemical Group, 800 N. Lindberg Blvd., St.
A
Louis, MO 63167. See Note 1.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5492
9.3.11.1 Long Precipitation Time—Allow the flask to stand
undisturbed 16 to 20 h at 25 6 0.5°C. Stir or gently shake the
flask to break up any precipitated-polymer gel at the end of the
precipitation time, or
9.3.11.2 Short-Precipitation Time—Without stirring, cool
the solution for 60 min in the temperature-controlled bath at
25 6 0.5°C. Use a timer with an alarm to ensure that the
solution-precipitation time ranges from 60 to 62 min. Remove
the flask from the temperature-controlled bath. Stir or gently
shake the flask to break up any precipitated-polymer gel.
NOTE 8—The long-precipitation method should be used only for
homopolymers and copolymers with solubles of less than 12 %. Results
have shown that copolymers with high levels of polymer solubles take as
much as three days to reach equilibrium, therefore long precipitation times
do not have any advantage over short precipitation times.
9.3.12 Place a 200-mm No. 4 filter paper or equivalent in a
200-mm funnel in a funnel rack over a 250-mL glass-stoppered
flask.
9.3.13 For each sample, pour the contents from the flask
into a funnel and allow the filtrate to drip into the second flask.
9.3.14 Continue the filtration until all the filtrate has been
collected. If the filtered solution is not completely clear it will
be necessary to repeat the filtration.
9.3.15 Dry the aluminum pans for 30 min in an oven at
150°C. Cool the pans in a desiccator until ready for use. For
each sample weigh a clean, dry pan on the analytical balance to
the nearest 0.0001 g.
FIG. 1 Equipment Setup
9.3.16 With the clean Class A pipette, pipet a 100-mL
aliquot of the soluble filtrate into the weighed aluminum pan.
9.3.17 Place the pan on a temperature-controlled hot plate
9.3.6 Blanket the contents of the boiling flask with a slow
maintained at 145 to 150°C. Allow the aliquot to obtain a
flow of nitrogen directed across the top of the condenser, not
rolling boil to prevent splashing. Blanket the pan with a slow
directly into the condenser, to minimize possible o-xylene loss.
stream of nitrogen. Continue heating the pan until the residue
Flow rate should be approximately 2 L/h.
in the dish is almost dry.
9.3.7 Heat the polymer/o-xylene mixture to reflux tempera-
9.3.18 Place the pan in a vacuum oven at 100 6 5°C for
ture while vigorously stirring. The stirring shall be vigorous
about an hour at a pressure less than 13.3 kPa.
enough to obtain a deep vortex, which keeps the boiling under
control and prevents boiling up into the condenser. This will 9.3.19 Cool the pan to room temperature in a desiccator and
weigh the pan to the nearest 0.0001 g. Repeat the drying,
take approximately 30 min. Ensure that the reflux is gentle so
that localized burning and sticking of the polymer to the flask cooling, and weighing steps until two consecutive weighings
agree within 0.0002 g.
walls does not occur.
9.3.8 Once the reflux temperature is reached, stir the solu- 9.3.20 Obtain the mass of the soluble fraction by subtracting
the mass of the pan from the residue-and-pan mass. Adjust the
tion for an additional 30 min. The liquid will appear water-
clear. final percent-soluble-fraction data to account for the percent
residual contamination from o-xylene.
9.3.9 Remove the heating plate from beneath the flask.
Detach the flask from the condenser and lightly stopper the
10. Calculation
flask. Cool the solution temperature from reflux temperature to
below 100°C by cooling the flask in air for 12 to 14 min. A
10.1 Calculate the mass percent of the soluble fraction by
timer with alarm must be used to ensure that the solution is
use of the following equation:
cooled to within a narrow range of temperature from run to run.
S 5~~V /V 3~W 2W !2V /V 3B!/W !3100
s bo b1 2 1 bo b2 o
9.3.10 Transfer the flask to a thermostatically controlled
water bath at 25 6 0.5°C. Shake the flask to break up any
where:
precipitate before immersing the flask in the bath.
S = soluble fraction of sample,
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.