ASTM B764-94(2003)
(Test Method)Standard Test Method for Simultaneous Thickness and Electrochemical Potential Determination of Individual Layers in Multilayer Nickel Deposit (STEP Test)
Standard Test Method for Simultaneous Thickness and Electrochemical Potential Determination of Individual Layers in Multilayer Nickel Deposit (STEP Test)
SCOPE
1.1 This test method closely estimates the thickness of individual layers of a multilayer nickel deposit and the electrochemical potential differences between the individual layers while being anodically stripped at constant current density.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: B 764 – 94 (Reapproved 2003)
Standard Test Method for
Simultaneous Thickness and Electrochemical Potential
Determination of Individual Layers in Multilayer Nickel
Deposit (STEP Test)
This standard is issued under the fixed designation B 764; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope serves as the bottom of the cell. To the bottom of the cell is
attached a rubber or plastic gasket whose opening defines the
1.1 This test method closely estimates the thickness of
measuring (stripping, anodic) area. If a metallic cell is used, the
individual layers of a multilayer nickel deposit and the elec-
rubber gasket also electrically insulates the test specimen from
trochemical potential differences between the individual layers
2,3
the cell. With the specimen as the anode and the cell or agitator
while being anodically stripped at constant current density.
tube as the cathode, a constant direct current is passed through
1.2 This standard does not purport to address all of the
the cell until the nickel layer is dissolved. A sudden change in
safety concerns, if any, associated with its use. It is the
voltage between the electrodes occurs when a different metallic
responsibility of the user of this standard to establish appro-
layer starts to dissolve.
priate safety and health practices and determine the applica-
3.3 Each different metal or species of the same metal
bility of regulatory limitations prior to use.
requires a given voltage (electrochemical potential) to keep the
2. Referenced Documents current constant while being stripped. As one nickel layer is
dissolved away and the next layer becomes exposed, there will
2.1 ASTM Standards:
be a voltage change (assuming a constant current and differ-
B 504 Test Method for Measurement of Thickness of Me-
ence in the electrochemical characteristics of the two nickel
tallic Coatings by the Coulometric Method
layers). The elapsed time at which this voltage change occurs
D 1193 Specification for Reagent Water
(relative to the start of the test or previous voltage change) is
3. Summary of Test Method
a measure of the deposit thickness.
3.4 At the same time, the amplitude of the voltage change
3.1 This procedure is a modification of the well-known
can be observed. That is, the ease (or difficulty) with which one
coulometric method of thickness testing (Test Method B 504).
layer can be dissolved or stripped with reference to another
It is also known as the anodic dissolution or electrochemical
layer can be compared. The lower the voltage needed the more
stripping method.
active the metal or the greater the tendency to corrode
3.2 Coulometric thickness testing instruments are based on
preferentially to a more noble metal adjacent to it.
the anodic dissolution (stripping) of the deposit at constant
3.5 Where the metallic layers are of such a similar nature
current, while the time is measured to determine thickness. As
that change of the stripping voltage is small, there can be
commonly practiced, the method employs a small cell that is
problems in detecting this change if the voltage between the
filled with an appropriate electrolyte, and the test specimen
deplating cell (cathode) and the sample (anode) is measured.
As the sample is dissolved anodically, cathodic processes are
This method is under the jurisdiction of ASTM Committee B08 on Metallic and
occurring on the deplating cell (cathode) surface that can also
Inorganic Coatings and is the direct responsibility of Subcommittee B08.10 on Test
give rise to voltage changes, due to alterations of the cathode
Methods.
surface, thus obscuring the anode voltage change. This diffi-
Current edition approved May 10, 2003. Published July 2003. Originally
approved in 1986. Last previous edition approved in 1994 as B 764 – 94. culty can be avoided by measuring the potential of the
For discussion of this test, see Harbulak, E. P., “Simultaneous Thickness and
dissolving anodic sample with respect to an unpolarized third
Electrochemical Potential Determination of Individual Layers in Multilayer Nickel
electrode (reference) placed in the cell. By recording this
Deposits,” Plating and Surface Finishing, Vol 67, No. 2, February 1980, pp. 49–54.
potential any difference in electrochemical activity between
U.S. Patent 4,310,389. Assignee: The Chrysler Corp., Highland Park, MI
48203.
layers is more readily detected.
Annual Book of ASTM Standards, Vol 02.05.
Annual Book of ASTM Standards, Vol 11.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
B 764 – 94 (2003)
3.6 The thickness of any specific nickel layer may be 5.4 Recorder—Any time-based recorder with an input im-
calculated from the quantity of electricity used (current multi- pedance of at least 1.0 MV and capable of running at
plied by time), the area dissolved, the electrochemical equiva- approximately 0.5 mm/s (3 cm/min) can be used.
lent of nickel, the anode efficiency, and the density of the nickel 5.5 Deplating Cell—The cell may be similar in construction
layer. to commercially available deplating cells. It is usually a
cup-shaped cell of either 316 stainless steel, Monel, or plastic
4. Significance and Use
that engages a round rubber or plastic gasket to the work piece
4.1 The ability of a multilayer nickel deposit to enhance
or sample. The opening through the cell and gasket allows
corrosion resistance is a function of the electrochemical contact of the electrolyte to the test specimen and defines the
potential differences between the layers (as measured individu-
stripping area.
ally at a fixed current density in a given electrolyte versus a
NOTE 2—A deplating cell could be constructed of plastic using a
reference electrode) and the thicknesses of the layers. The
cylindrical stainless steel or Monel sheet cathode located in the larger
potential differences must be sufficient to cause the bright
upper area of the cup. The advantages of such a cell are the prevention of
nickel or top layer to corrode preferentially and sacrificially
whisker growth and the choking off of the small bore opening, and the
with respect to the semibright nickel layer beneath it. ease of cathode removal for cleaning or replacement.
4.2 This test procedure allows the measurement of these
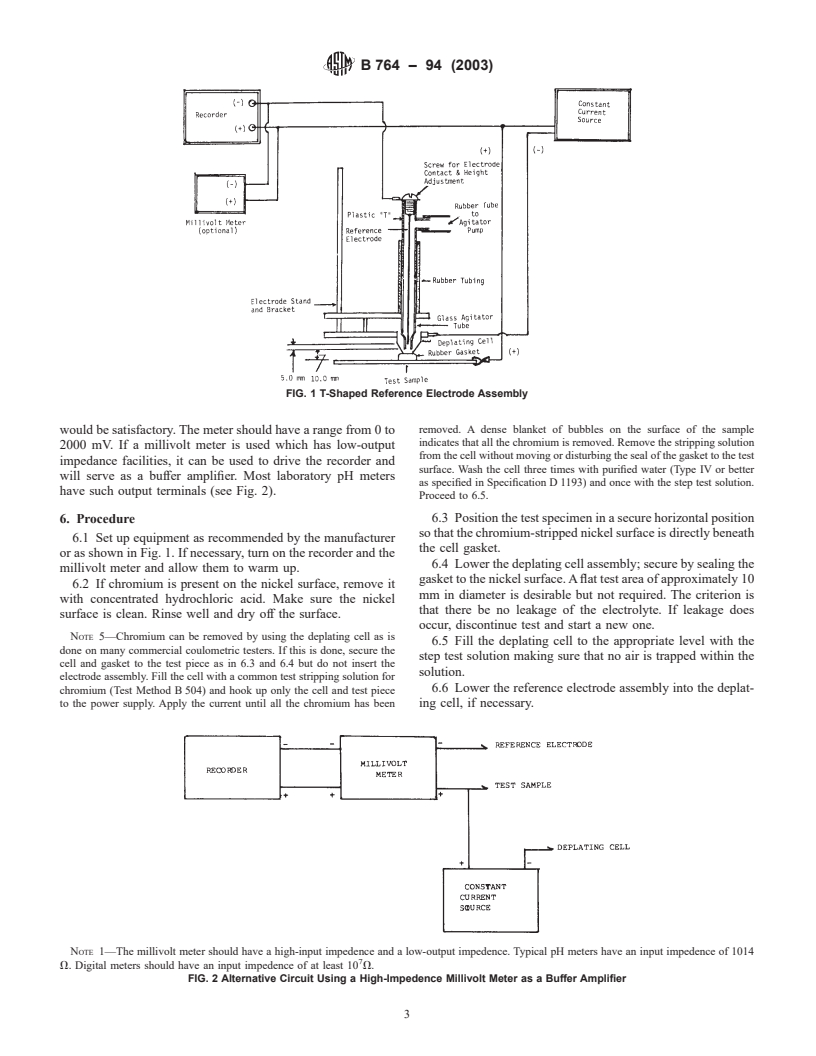
5.6 Reference Electrode Assembly—The general configura-
potential differences directly on an electroplated part rather
tion of one commercially available measuring system is shown
than on separate foil specimens in such a way that time
in Fig. 1. The assembly is a “T”-shaped housing containing the
determines the thickness of each layer, while the potential
reference electrode. The housing assembly includes an upper
difference between nickel layers is an indication of the corro-
connector providing a port for the connection to the agitator
sion resistance of the total nickel deposit.
pump. The upper position also contains a threaded contact to
4.3 The interpretation and evaluation of the results of this
the reference electrode. The lower portion of the assembly
test should be by agreement between the purchaser and the
contains an adjustable glass or plastic tube which surrounds the
manufacturer.
reference electrode so that when inserted into the deplating
cell, the reference electrode is immersed in the electrolyte. The
NOTE 1—This test may be used to help predetermine the corrosion
resistance of the multilayer nickel coatings applied in production that are tip of the reference electrode should extend into the glass tip so
subjected to other corrosion media. It should be understood that due to
that the distance between the tip of the electrode and the
many factors that influence the progress of corrosion during actual use of
bottom of the glass agitator tube is approximately 5.0 mm.
the part, the performance of different multilayer nickel deposits in the test
5.7 Reference Electrode—Either silver or platinum wire of
cannot be taken as an absolute indicator of the relative corrosion resistance
approximately 1.5 mm in diameter can be used. Silver is
of these deposits in service.
probably the better choice due to its ability to form a
5. Apparatus silver-silver chloride electrode when used in a chloride con-
taining electrolyte (see Appendix X1).
5.1 Composition of the Electrolyte:
NOTE 3—It is necessary to condition the silver electrode before using in
order to form the silver-silver chloride surface. This is easily done by
Nickel Chloride (NiCl ·6H O) 300g/L
2 2
Sodium Chloride (NaCl) 50g/L anodically treating approximately a 75-mm length of wire in an 1 N
Boric Acid (H BO ) 25g/L
3 3 hydrochloric acid solution for 10 to 15 s using a 34.7-mA anodic current.
A
pH 3.0
This will form a gray film on the wire which should always be present.
Once the gray film is formed, it is not necessary to repeat the conditioning
A
The pH may be adjusted with diluted hydrochloric acid or sodium hydroxide, as
treatment unless the film has been removed. It may be advisable, however,
required, and is more critical than the composition of the electrolyte.
to recondition the electrode after a prolonged period of inactivity or when
Prepared in Purified Water—Type IV or better as specified
the electrode has been allowed to remain dry for an extended period of
in Specification D 1193.
time. Drying off the electrode should be avoided by immersion in either
5.2 Constant Current Source—This should supply a con-
the hydrochloric acid conditioning solution, the step test solution, or
stant current that can be varied between 0 and 50 mA (typical
distilled water when not in use.
25 to 35 mA). A current of 30 mA corresponds to a stripping
NOTE 4—A ceramic junction reference electrode that does not require
rate of 7.8 μm/min at 100 % current efficiency when used with conditioning is available commercially.
a gasket providing 0.08 cm stripping area. (This is achieved
5.8 Millivolt Meter (optional)—When using a sensitive and
with the solution stated in 5.1.) Most commercial coulometric
well-calibrated recorder, a millivolt meter is not necessary. If
thickness testers can be used with slight modification as the
one is desired, however, any sensitive, high-input impedance
current source.
meter can be used. A standard pH meter with a millivolt setting
5.3 Electrolyte Agitation Source—All commercial coulom-
etric thickness testers incorporate a means to agitate the
solution. It is possible to purchase these types of units
Monel is a trademark for a large group of corrosion-resistant alloys of
separately, if so desired, to be used externally in conjunction
predominantly nickel and copper. Available from Huntington Alloy Products Div.,
with other power supplies. The International Nickel Co., Inc., Huntington, WV 25720.
B 764 – 94 (2003)
FIG. 1 T-Shaped Reference Electrode Assembly
removed. A dense blanket of bubbles on the surface of the sample
would be satisfactory. The meter should have a range from 0 to
indicates that all the chromium is removed. Remove the stripping solution
2000 mV. If a millivolt meter is used which has low-output
from the cell without moving or disturbing the seal of the gasket to the test
impedance facilities, it can be used to drive the recorder and
surface. Wash the cell three times with purified water (Type IV or better
will serve as a buffer amplifier. Most laboratory pH meters
as specified in Specification D 1193) and once with the step test solution.
have such output terminals (see Fig. 2).
Proceed to 6.5.
6. Procedure 6.3 Position the test specimen in a secure horizontal position
so that the chromium-stripped nickel surface is directly beneath
6.1 Set up equipment as recommended by the manufacturer
the cell gasket.
or as shown in Fig. 1. If necessary, turn on the recorder and the
6.4 Lower the deplating cell assembly; secure by sealing the
millivolt meter and allow them to warm up.
gasket to the nickel surface. A flat test area of approximately 10
6.2 If chromium is present on the nickel surface, remove it
mm in diameter is desirable but not required. The criterion is
with concentrated hydrochloric acid. Make sure the nickel
that there be no leakage of the electrolyte. If leakage does
surface is clean. Rinse well and dry off the surface.
occur, discontinue test and start a new one.
NOTE 5—Chromium can be removed by using the deplating cell as is
6.5 Fill the deplating cell to the appropriate level with the
done on many commercial coulometric testers. If this is done, secure the
step test solution making sure that no air is trapped within the
cell and gasket to the test piece as in 6.3 and 6.4 but do not insert the
solution.
electrode assembly. Fill the cell with a common test stripping solution for
6.6 Lower the reference electrode assembly into the deplat-
chromium (Test Method B 504) and hook up only the cell and test piece
to the power supply. Apply the current until all the chromium has been ing cell, if necessary.
NOTE 1—The millivolt meter should have a high-input impedence and a low-output impedence. Typical pH meters have an input impedence of 1014
V. Digital meters should have an input impedence of at least 10 V.
FIG. 2 Alternative Circuit Using a High-Impedence Millivolt Meter as a Buffer Amplifier
B 764 – 94 (2003)
NOTE 6—The insertion depth of the electrolyte agitation tube which
where:
includes the reference electrode is important and should always be the
SL = chart scan length, mm,
same. The positioning of the reference electrode should be such that the
S = chart speed, mm/min,
distance from the end of the electrode to the test specimen is reproducible
I = cell current, mA,
to within 1 mm and be held constant throughout the test. It should be
A = deplating area, cm, and
remembered that the difference of potential rather than the absolute
T = nickel thickness, μm. Constant (0.303) is calculated
potential is the important measurement.
from the electrochemical equivalent and density of
6.7 Check all electrical connections. Make sure all connec-
nickel.
tions are secure and that no corrosion exists at the contact
6.9 Turn on the constant current source and agitator which
points and that all contact points are secure.
in turn will start the deplating reaction. Continue recording
6.8 Start the recorder (turn on milliampere meter, if used).
until the surface underlying the nickel is reached. This end
The recorder must be calibrated in order to determine the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.