ASTM E2699-10
(Practice)Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Digital Radiographic (DR) Test Methods
Standard Practice for Digital Imaging and Communication in Nondestructive Evaluation (DICONDE) for Digital Radiographic (DR) Test Methods
SIGNIFICANCE AND USE
Personnel that are responsible for the creation, transfer, and storage of digital X-ray test results will use this standard. This practice defines a set of information modules that along with Practice E2339 and the DICOM standard provide a standard means to organize digital X-ray test parameters and results. The digital X-ray test results may be displayed and analyzed on any device that conforms to this standard. Personnel wishing to view any digital X-ray inspection data stored according to Practice E2339 may use this document to help them decode and display the data contained in the DICONDE-compliant inspection record.
SCOPE
1.1 This practice facilitates the interoperability of digital X-ray imaging equipment by specifying image data transfer and archival methods in commonly accepted terms. This document is intended to be used in conjunction with Practice E2339 on Digital Imaging and Communication in Nondestructive Evaluation (DICONDE). Practice E2339 defines an industrial adaptation of the NEMA Standards Publication titled Digital Imaging and Communications in Medicine (DICOM, see http://medical.nema.org), an international standard for image data acquisition, review, storage and archival storage. The goal of Practice E2339, commonly referred to as DICONDE, is to provide a standard that facilitates the display and analysis of NDE results on any system conforming to the DICONDE standard. Toward that end, Practice E2339 provides a data dictionary and a set of information modules that are applicable to all NDE modalities. This practice supplements Practice E2339 by providing information object definitions, information modules and a data dictionary that are specific to digital X-ray test methods.
1.2 This practice has been developed to overcome the issues that arise when analyzing or archiving data from digital X-ray equipment test using proprietary data transfer and storage methods. As digital technologies evolve, data must remain decipherable through the use of open, industry-wide methods for data transfer and archival storage. This practice defines a method where all the digital X-ray technique parameters and test results are communicated and stored in a standard manner regardless of changes in digital technology.
1.3 This practice does not specify:
1.3.1 A testing or validation procedure to assess an implementation's conformance to the standard.
1.3.2 The implementation details of any features of the standard on a device claiming conformance.
1.3.3 The overall set of features and functions to be expected from a system implemented by integrating a group of devices each claiming DICONDE conformance.
1.3.4 Although this practice contains no values that require units, it does describe methods to store and communicate data that do require units to be properly interpreted. The SI units required by this practice are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2699 – 10
Standard Practice for
Digital Imaging and Communication in Nondestructive
Evaluation (DICONDE) for Digital Radiographic (DR) Test
Methods
This standard is issued under the fixed designation E2699; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.3.3 The overall set of features and functions to be ex-
pected from a system implemented by integrating a group of
1.1 This practice facilitates the interoperability of digital
devices each claiming DICONDE conformance.
X-ray imaging equipment by specifying image data transfer
1.3.4 Although this practice contains no values that require
and archival methods in commonly accepted terms. This
units, it does describe methods to store and communicate data
document is intended to be used in conjunction with Practice
that do require units to be properly interpreted. The SI units
E2339 on Digital Imaging and Communication in Nondestruc-
required by this practice are to be regarded as standard. No
tive Evaluation (DICONDE). Practice E2339 defines an indus-
other units of measurement are included in this standard.
trial adaptation of the NEMA Standards Publication titled
1.4 This standard does not purport to address all of the
Digital Imaging and Communications in Medicine (DICOM,
safety concerns, if any, associated with its use. It is the
see http://medical.nema.org), an international standard for
responsibility of the user of this standard to establish appro-
image data acquisition, review, storage and archival storage.
priate safety and health practices and determine the applica-
The goal of Practice E2339, commonly referred to as DI-
bility of regulatory limitations prior to use.
CONDE,istoprovideastandardthatfacilitatesthedisplayand
analysis of NDE results on any system conforming to the
2. Referenced Documents
DICONDEstandard.Towardthatend,PracticeE2339provides
2.1 ASTM Standards:
a data dictionary and a set of information modules that are
E1316 Terminology for Nondestructive Examinations
applicable to all NDE modalities. This practice supplements
E1475 Guide for Data Fields for Computerized Transfer of
Practice E2339 by providing information object definitions,
Digital Radiological Examination Data
information modules and a data dictionary that are specific to
E2339 Practice for Digital Imaging and Communication in
digital X-ray test methods.
Nondestructive Evaluation (DICONDE)
1.2 This practice has been developed to overcome the issues
E2597 Practice for Manufacturing Characterization of Digi-
that arise when analyzing or archiving data from digital X-ray
tal Detector Arrays
equipment test using proprietary data transfer and storage
2.2 Other Standard:
methods. As digital technologies evolve, data must remain
DICOM National Electrical Manufacturers Association
decipherable through the use of open, industry-wide methods
Standard for Digital Imaging and Communications in
for data transfer and archival storage. This practice defines a
Medicine (DICOM), 2008
method where all the digital X-ray technique parameters and
test results are communicated and stored in a standard manner
3. Terminology
regardless of changes in digital technology.
3.1 Definitions:
1.3 This practice does not specify:
3.1.1 Nondestructive evaluation terms used in this practice
1.3.1 A testing or validation procedure to assess an imple-
can be found in Standard Terminology for Nondestructive
mentation’s conformance to the standard.
Examinations, E1316.
1.3.2 The implementation details of any features of the
standard on a device claiming conformance.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
This practice is under the jurisdiction of ASTM Committee E07 on Nonde- contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
structive Testing and is the direct responsibility of Subcommittee E07.11 on Digital Standards volume information, refer to the standard’s Document Summary page on
Imaging and Communication in Nondestructive Evaluation (DICONDE). the ASTM website.
Current edition approved June 1, 2010. Published June 2010. DOI:10.1520/ Available from National Electrical Manufacturers Association (NEMA), 1300
E2699-10. N. 17th St., Suite 1752, Rosslyn, VA 22209, http://www.nema.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E2699 – 10
3.1.2 DICONDE terms used in this practice are defined in digital X-ray imaging device for NDE purposes. To avoid
Practice E2339. duplication of relevant material from the DICOM standard, the
3.1.3 Digital detector array terms used in this practice are IOD definition will follow that for DX Images found in Part 3,
defined in Practice E2597. sectionA.26 of the DICOM standard except as noted below in
Table 1. Table 1 is not stand-alone and must be used in
4. Summary of Practice
conjunction with Part 3, section A.26 of the DICOM standard
4.1 A fundamental principle of DICONDE is the use of
to have a complete definition of the NDE DX information
standard definitions and attribute formats for data communica-
object. This IOD will use the Service-Object Pair (SOP)
tion and storage. This means all systems that are DICONDE
Classes for the DX IOD as defined in Part 4 of the DICOM
compliant use a common data dictionary and common com-
standard.
munication protocols. To further standardization, the elements
6.2 Digital X-ray Multi-Frame Image IOD Description:
in the data dictionary are organized into common groups
6.2.1 The digital X-ray (DX) Multi-frame (MF) Image
referred to as information modules. The data dictionary and
Information Object Definition specifies an image that has been
information modules common to all NDE modalities are
created by a direct digital X-ray imaging device for NDE
defined in Practice E2339.
purposes. To avoid duplication of relevant material from the
4.2 The data dictionary and information modules specified
DICOM standard, the IOD definition will follow that for
in Practice E2339 do not cover the information storage
Enhanced X-ray Angiographic (Enhanced XA) Images found
requirements for each individual modality (CT, DR, CR, UT,
in Part 3, sectionA.47 of the DICOM standard except as noted
etc.).Additions to the data dictionary and information modules
below in Table 2. Table 2 is not stand-alone and must be used
are required to support the individual modalities. This practice
in conjunction with Part 3, section A.47 of the DICOM
contains the additions to the DICONDE data dictionary and
standard to have a complete definition of the NDE DX-MF
information modules necessary for digital X-ray inspection.
information object. This IOD will use the Service-Object Pair
4.3 The highest organizational level in the DICONDE
(SOP) Classes for the Enhanced XA IOD as defined in Part 4
information model is the information object definition (IOD).
of the standard.
An information object definition is a collection of the infor-
mation modules necessary to represent a set of test results from
7. Information Modules
a specific modality. This practice contains information object
7.1 NDE DX Detector Module:
definitions for digital X-ray inspection.
7.1.1 Table 3 specifies the Attributes that describe NDE
5. Significance and Use
Direct Digital X-ray (DX) Detectors.
5.1 Personnel that are responsible for the creation, transfer, 7.1.1.1 For NDE DX Images, Detector Type (0018,7004) is
and storage of digital X-ray test results will use this standard. specified to use the following defined terms.
This practice defines a set of information modules that along
DIRECT
with Practice E2339 and the DICOM standard provide a
SCINTILLATOR
standard means to organize digital X-ray test parameters and
7.1.1.2 For NDE DX Images, Detector Configuration
results. The digital X-ray test results may be displayed and
(0018,7005) is specified to use the following defined terms.
analyzed on any device that conforms to this standard. Person-
AREA
nel wishing to view any digital X-ray inspection data stored
LINEAR
according to Practice E2339 may use this document to help
7.2 NDE DX Calibration Data Module:
them decode and display the data contained in the DICONDE-
7.2.1 Table 4 specifies the Attributes that describe NDE
compliant inspection record.
direct digital X-ray calibration data.
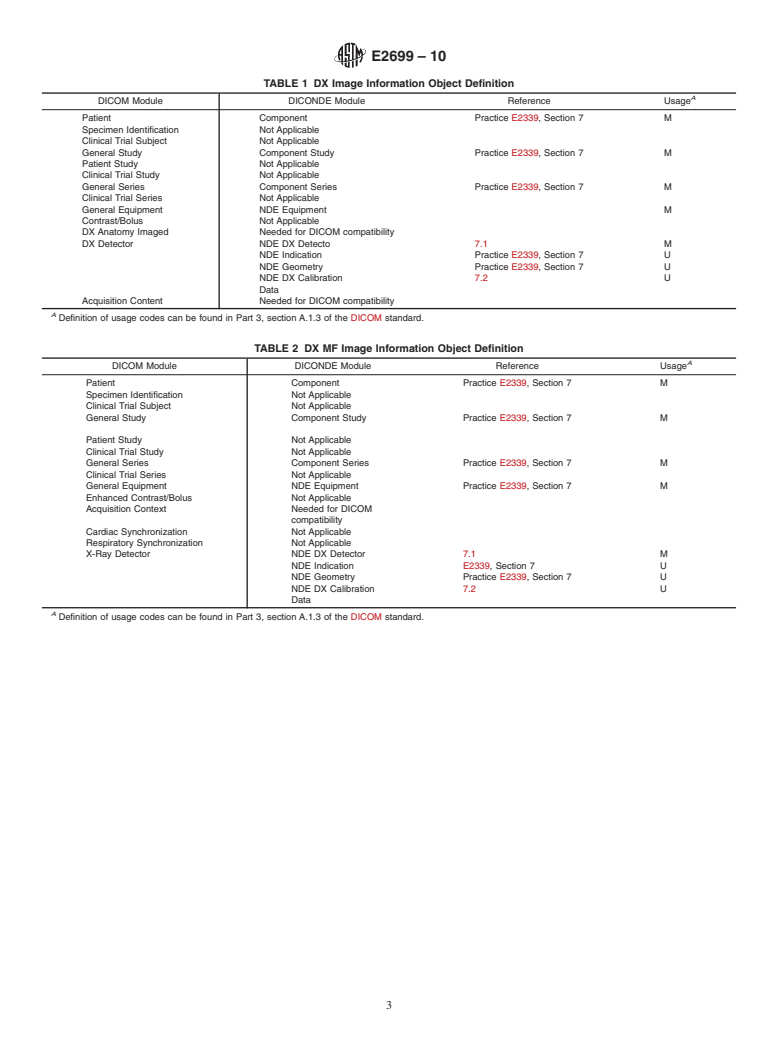
6. Information Object Definitions
8. Keywords
6.1 Digital X-ray Image IOD Description:
6.1.1 The digital X-ray (DX) Image Information Object 8.1 DICOM; DICONDE; direct digital X-ray; DX; digital
Definition specifies an image that has been created by a direct data transmission; digital data storage; database; file format
E2699 – 10
TABLE 1 DX Image Information Object Definition
A
DICOM Module DICONDE Module Reference Usage
Patient Component Practice E2339, Section 7 M
Specimen Identification Not Applicable
Clinical Trial Subject Not Applicable
General Study Component Study Practice E2339, Section 7 M
Patient Study Not Applicable
Clinical Trial Study Not Applicable
General Series Component Series Practice E2339, Section 7 M
Clinical Trial Series Not Applicable
General Equipment NDE Equipment M
Contrast/Bolus Not Applicabl
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.