ASTM D7620-10(2015)
(Test Method)Standard Test Method for Determination of Total Sulfur in Liquid Hydrocarbon Based Fuels by Continuous Injection, Air Oxidation and Ultraviolet Fluorescence Detection
Standard Test Method for Determination of Total Sulfur in Liquid Hydrocarbon Based Fuels by Continuous Injection, Air Oxidation and Ultraviolet Fluorescence Detection
SIGNIFICANCE AND USE
5.1 Some process catalysts used in refining can be poisoned when trace amounts of sulfur bearing materials are contained in the feedstocks. There are also government regulations as to how much sulfur is permitted to be present in commercial transportation fuels. This test method can be used to determine sulfur in process and downstream distribution streams. It can also be used for purposes of screening and quality control of finished hydrocarbon fuel products.
SCOPE
1.1 This test method covers the determination of total sulfur in liquid hydrocarbon based fuel with a final boiling point of up to 450 °C. It is applicable to analysis of natural, processed and final product materials containing sulfur in the range of 4.0 mg/kg to 830 mg/kg (see Note 1).
Note 1: For liquid hydrocarbons containing less than 4.0 mg/kg total sulfur or more than 830 mg/kg total sulfur, Test Method D5453 may be more appropriate.
1.2 This test method is applicable for total sulfur determination in liquid hydrocarbons containing less than 0.35 % (m/m) halogen(s).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see 4.1, 8.3, and Section 9.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D7620 − 10 (Reapproved 2015)
Standard Test Method for
Determination of Total Sulfur in Liquid Hydrocarbon Based
Fuels by Continuous Injection, Air Oxidation and Ultraviolet
Fluorescence Detection
This standard is issued under the fixed designation D7620; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D4175 Terminology Relating to Petroleum, Petroleum
Products, and Lubricants
1.1 This test method covers the determination of total sulfur
D4177 Practice for Automatic Sampling of Petroleum and
inliquidhydrocarbonbasedfuelwithafinalboilingpointofup
Petroleum Products
to 450 °C. It is applicable to analysis of natural, processed and
D5453 Test Method for Determination of Total Sulfur in
final product materials containing sulfur in the range of
Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel
4.0 mg⁄kg to 830 mg⁄kg (see Note 1).
Engine Fuel, and Engine Oil by Ultraviolet Fluorescence
NOTE 1—For liquid hydrocarbons containing less than 4.0 mg⁄kg total
D6299 Practice for Applying Statistical Quality Assurance
sulfur or more than 830 mg⁄kg total sulfur, Test Method D5453 may be
and Control Charting Techniques to Evaluate Analytical
more appropriate.
Measurement System Performance
1.2 This test method is applicable for total sulfur determi-
D6300 Practice for Determination of Precision and Bias
nation in liquid hydrocarbons containing less than 0.35 %
Data for Use in Test Methods for Petroleum Products and
(m⁄m) halogen(s).
Lubricants
D6792 Practice for Quality System in Petroleum Products
1.3 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this and Lubricants Testing Laboratories
standard.
3. Terminology
1.4 This standard does not purport to address all of the
3.1 Definitions:
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- 3.1.1 See Terminology D4175 for definitions of other terms
priate safety and health practices and determine the applica- used in this test method.
bility of regulatory limitations prior to use. For specific hazard 3.1.2 oxidative pyrolysis, n—process in which a sample
statements, see 4.1, 8.3, and Section 9. undergoes complete combustion in an appropriate oxygen
containing environment at a sufficiently elevated temperature.
2. Referenced Documents
3.1.2.1 Discussion—Organic compounds pyrolytically oxi-
dize to carbon dioxide and water and oxides of other elements
2.1 ASTM Standards:
D1298 Test Method for Density, Relative Density, or API that are in the sample.
Gravity of Crude Petroleum and Liquid Petroleum Prod-
4. Summary of Test Method
ucts by Hydrometer Method
D4052 Test Method for Density, Relative Density, and API
4.1 A small, very controlled flow of hydrocarbon sample is
Gravity of Liquids by Digital Density Meter
continuously injected during measurement. It is introduced via
D4057 Practice for Manual Sampling of Petroleum and
a syringe into a high temperature combustion tube containing
Petroleum Products
air where sulfur is oxidized to sulfur dioxide (SO ). Water
produced during the sample combustion is removed, as
This test method is under the jurisdiction of ASTM Committee D02 on
required, and the sample combustion gases are next exposed to
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
ultraviolet (UV) light. The SO absorbs the energy from the
Subcommittee D02.03 on Elemental Analysis.
UV light and is converted to excited sulfur dioxide (SO *).
Current edition approved July 1, 2015. Published July 2015. Originally approved
Fluorescence emitted from the excited SO * as it returns to a
in 2010. Last previous edition approved in 2010 as D7620 – 10. DOI:10.1520/
D7620-10R15.
stable state SO is detected by a photomultiplier tube and the
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
resulting signal is a measure of the sulfur contained in the
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
sample. (Warning—Exposure to excessive quantities of ultra-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. violet light is injurious to health. The operator shall avoid
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D7620 − 10 (2015)
FIG. 1 Basic Block Diagram Describing Sulfur Determination
exposing their body, especially their eyes, not only to direct
UV light but also to secondary or scattered radiation that is
present.)
4.2 Fig. 1 illustrates a basic block diagram describing sulfur
determination. Sample collection and conditioning, sample
introduction, detection system and data handling are depicted.
5. Significance and Use
5.1 Some process catalysts used in refining can be poisoned
whentraceamountsofsulfurbearingmaterialsarecontainedin
the feedstocks. There are also government regulations as to
how much sulfur is permitted to be present in commercial
transportation fuels. This test method can be used to determine
sulfur in process and downstream distribution streams. It can
also be used for purposes of screening and quality control of
finished hydrocarbon fuel products.
6. Interferences
6.1 Halogens above 0.35 % (mass/mass) will interfere with
accurate sulfur determination.
6.2 Bound nitrogen at concentration greater than 150 mg
N/kg can cause a 1 mg S/kg positive bias.
6.3 Excessive moisture produced during the combustion
step will interfere if not removed prior to the detector.
7. Apparatus
7.1 Furnace—An electric furnace held at a temperature
sufficient to pyrolyze the entire sample (typically 1050 °C 6
25 °C) and oxidize sulfur to SO .
7.2 Combustion Tube—A quartz combustion tube con-
structed to allow the direct injection of a continuous flow of
sample into the heated oxidation zone of the furnace. The
oxidation section shall be large enough to ensure complete
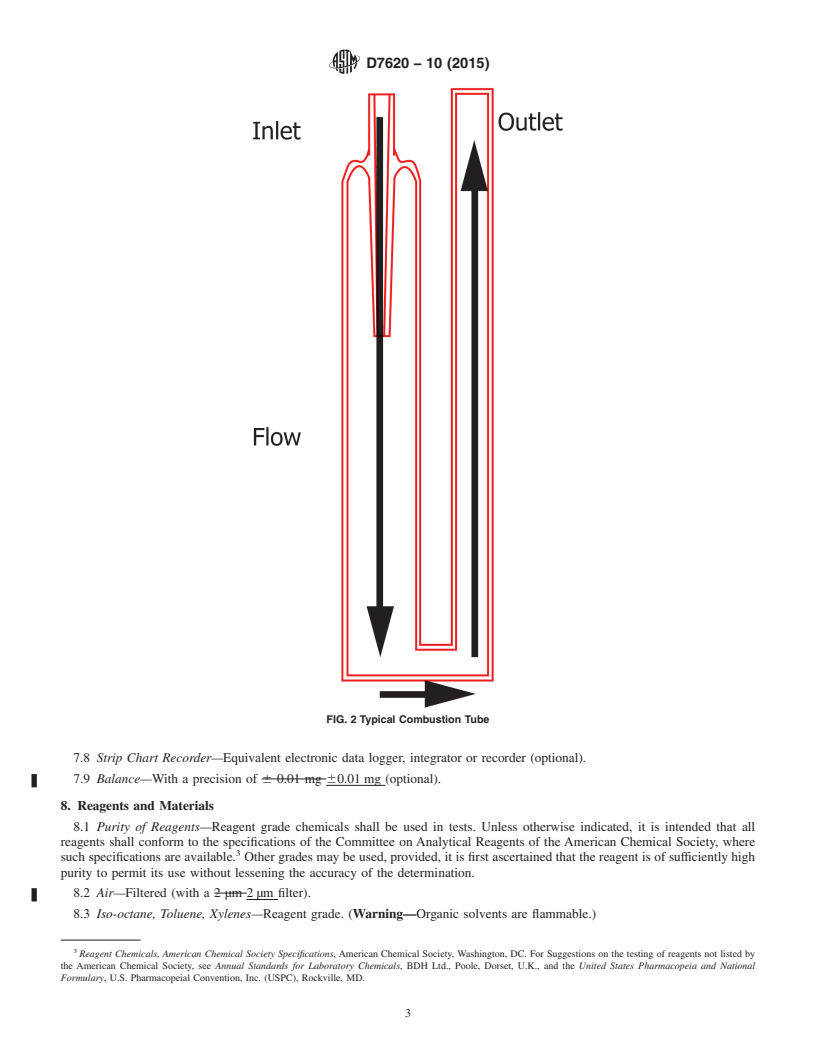
combustion of the sample. Fig. 2 illustrates a typical combus-
tion tube (Note 2).
NOTE 2—Other combustion tube configurations are acceptable if
precision and accuracy are not degraded.
7.3 Flow Control—The apparatus shall be equipped with
suitable flow control apparatus capable of maintaining a
FIG. 2 Typical Combustion Tube
constant supply of air.
7.4 Drier Tube—The apparatus shall be equipped with a
mechanism for the removal of excessive water vapor. The accomplished with a membrane drying tube, or a permeation
oxidation reaction produces water vapor which must be elimi- dryer, that utilizes a selective capillary action for water
nated prior to measurement by the detector. This may be removal.
D7620 − 10 (2015)
TABLE 1 Typical Operating Conditions
7.5 UV Fluorescence Detector—A quantitative detector ca-
pableofmeasuringtheenergyemittedfromthefluorescenceof Furnace Temperature 1050 °C
Furnace Pressure 28 kPa
sulfur dioxide by UV light.
Photo Multiplier Tube (PMT) 40 °C
Temperature
7.6 Millilitre Syringe—A disposable 1 mL syringe capable
PMT Voltage 900 V
of accurately delivering a controlled and constant flow of
Optics Purge Flow 80 mL ⁄ min to 100 mL ⁄ min
calibration and sample materials. The syringe shall accommo-
date a disposable tip to aid the filling of the syringe and a
disposable septum seal to accommodate penetration and
9. Hazards
sample flow.
9.1 Consult current OSHA regulations, suppliers’ Material
7.7 Sample Inlet System—An automatic sample injection
Safety Data Sheets, and local regulations for all materials used
device that is compatible with a disposable 1 mL syringe is
in this test method.
required. The injector shall allow the introduction of an
9.2 High temperature is employed in this test method.
appropriate continuous flow of sample into a combustion tube
Exercise extra care when using flammable materials near the
carrierstream,whichdirectsthesampleintotheoxidationzone
oxidative pyrolysis furnace.
at a controlled and repeatable rate.
9.3 Duetothetypesofsamplesanalyzedinthistestmethod,
7.8 Strip Chart Recorder—Equivalent electronic data
chemicalresistantglovesshouldbewornwhenperformingthis
logger, integrator or recorder (optional).
test method.
7.9 Balance—With a precision of 60.01 mg (optional).
10. Sampling
8. Reagents and Materials
10.1 CollectthesamplesinaccordancewithPracticeD4057
8.1 Purity of Reagents—Reagent grade chemicals shall be
or Practice D4177. To preserve volatile components which are
used in tests. Unless otherwise indicated, it is intended that all
in some samples, do not uncover samples any longer than
reagents shall conform to the specifications of the Committee
necessary.
on Analytical Reagents of the American Chemical Society,
where such specifications are available. Other grades may be
11. Preparation of Apparatus
used, provided, it is first ascertained that the reagent is of
11.1 Place the analyzer in service in accordance with the
sufficiently high purity to permit its use without lessening the
manufacturer’s instructions.
accuracy of the determination.
11.2 Typical instrument parameters are listed in Table 1.
8.2 Air—Filtered (with a 2 µm filter).
11.3 Prepare the sample introduction accessories, if
8.3 Iso-octane, Toluene, Xylenes—Reagent grade.
required, according to the manufacturer’s instructions.
(Warning—Organic solvents are flammable.)
11.4 Adjust the instrument sensitivity and baseline stability
8.4 Thiophene—FW84.14, Sulfur content 38.10 % (m/m).
and perform instrument blanking procedure following manu-
8.4.1 Other sources of sulfur and diluent materials may be
facturer’s guidelines.
used if precision and accuracy are not degraded.
8.4.2 Apply the appropriate correction for chemical impu-
12. Calibration
rity.
12.1 Choose which type of calibration method is required
8.5 Sulfur Stock Solution—1000 µg S/mL. Prepare a stock
(mass/volume or mass/mass), and prepare a calibration stan-
solution by accurately weighing 0.2624 g 6 0.013 g of thio-
dard from the stock solution (8.5) by volume or mass dilution.
phene into a tared 100 mL volumetric flask. Dilute to volume
12.2 Ifamass/massanalysisisbeingdonewitha
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D7620 − 10 D7620 − 10 (Reapproved 2015)
Standard Test Method for
Determination of Total Sulfur in Liquid Hydrocarbon Based
Fuels by Continuous Injection, Air Oxidation and Ultraviolet
Fluorescence Detection
This standard is issued under the fixed designation D7620; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the determination of total sulfur in liquid hydrocarbon based fuel with a final boiling point of up
to 450°C.450 °C. It is applicable to analysis of natural, processed and final product materials containing sulfur in the range of
4.04.0 mg ⁄kg to 830830 mg ⁄ mg/kg kg (see Note 1).
NOTE 1—For liquid hydrocarbons containing less than 4.04.0 mg ⁄ mg/kg kg total sulfur or more than 830830 mg ⁄ mg/kg kg total sulfur, Test Method
D5453 may be more appropriate.
1.2 This test method is applicable for total sulfur determination in liquid hydrocarbons containing less than 0.35% (m/m)0.35 %
(m ⁄m) halogen(s).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. For specific hazard statements, see 4.1, 8.3, and Section 9.
2. Referenced Documents
2.1 ASTM Standards:
D1298 Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by
Hydrometer Method
D4052 Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter
D4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D4175 Terminology Relating to Petroleum, Petroleum Products, and Lubricants
D4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
D5453 Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel,
and Engine Oil by Ultraviolet Fluorescence
D6299 Practice for Applying Statistical Quality Assurance and Control Charting Techniques to Evaluate Analytical Measure-
ment System Performance
D6300 Practice for Determination of Precision and Bias Data for Use in Test Methods for Petroleum Products and Lubricants
D6792 Practice for Quality System in Petroleum Products and Lubricants Testing Laboratories
3. Terminology
3.1 Definitions:
3.1.1 See Terminology D4175 for definitions of other terms used in this test method.
3.1.2 oxidative pyrolysis, n—process in which a sample undergoes complete combustion in an appropriate oxygen containing
environment at a sufficiently elevated temperature.
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcommittee
D02.03 on Elemental Analysis.
Current edition approved Sept. 1, 2010July 1, 2015. Published September 2010July 2015. DOI:10.1520/D7620–10.Originally approved in 2010. Last previous edition
approved in 2010 as D7620 – 10. DOI:10.1520/D7620-10R15.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D7620 − 10 (2015)
FIG. 1 Basic Block Diagram Describing Sulfur Determination
3.1.2.1 Discussion—
Organic compounds pyrolytically oxidize to carbon dioxide and water and oxides of other elements that are in the sample.
4. Summary of Test Method
4.1 A small, very controlled flow of hydrocarbon sample is continuously injected during measurement. It is introduced via a
syringe into a high temperature combustion tube containing air where sulfur is oxidized to sulfur dioxide (SO ). Water produced
during the sample combustion is removed, as required, and the sample combustion gases are next exposed to ultraviolet (UV) light.
The SO absorbs the energy from the UV light and is converted to excited sulfur dioxide (SO *). Fluorescence emitted from the
2 2
excited SO * as it returns to a stable state SO is detected by a photomultiplier tube and the resulting signal is a measure of the
2 2
sulfur contained in the sample. (Warning—Exposure to excessive quantities of ultraviolet light is injurious to health. The operator
shall avoid exposing their body, especially their eyes, not only to direct UV light but also to secondary or scattered radiation that
is present.)
4.2 Fig. 1 illustrates a basic block diagram describing sulfur determination. Sample collection and conditioning, sample
introduction, detection system and data handling are depicted.
5. Significance and Use
5.1 Some process catalysts used in refining can be poisoned when trace amounts of sulfur bearing materials are contained in
the feedstocks. There are also government regulations as to how much sulfur is permitted to be present in commercial
transportation fuels. This test method can be used to determine sulfur in process and downstream distribution streams. It can also
be used for purposes of screening and quality control of finished hydrocarbon fuel products.
6. Interferences
6.1 Halogens above 0.35 % (mass/mass) will interfere with accurate sulfur determination.
6.2 Bound nitrogen at concentration greater than 150 mg 150 mg N/kg can cause a 1 mg 1 mg S/kg positive bias.
6.3 Excessive moisture produced during the combustion step will interfere if not removed prior to the detector.
7. Apparatus
7.1 Furnace—An electric furnace held at a temperature sufficient to pyrolyze the entire sample (typically 10501050 °C 6
25°C)25 °C) and oxidize sulfur to SO .
7.2 Combustion Tube—A quartz combustion tube constructed to allow the direct injection of a continuous flow of sample into
the heated oxidation zone of the furnace. The oxidation section shall be large enough to ensure complete combustion of the sample.
Fig. 2 illustrates a typical combustion tube (Note 2).
NOTE 2—Other combustion tube configurations are acceptable if precision and accuracy are not degraded.
7.3 Flow Control—The apparatus shall be equipped with suitable flow control apparatus capable of maintaining a constant
supply of air.
7.4 Drier Tube—The apparatus shall be equipped with a mechanism for the removal of excessive water vapor. The oxidation
reaction produces water vapor which must be eliminated prior to measurement by the detector. This may be accomplished with
a membrane drying tube, or a permeation dryer, that utilizes a selective capillary action for water removal.
7.5 UV Fluorescence Detector—A quantitative detector capable of measuring the energy emitted from the fluorescence of sulfur
dioxide by UV light.
7.6 Millilitre Syringe—A disposable 1 mL 1 mL syringe capable of accurately delivering a controlled and constant flow of
calibration and sample materials. The syringe shall accommodate a disposable tip to aid the filling of the syringe and a disposable
septum seal to accommodate penetration and sample flow.
7.7 Sample Inlet System—An automatic sample injection device that is compatible with a disposable 1 mL 1 mL syringe is
required. The injector shall allow the introduction of an appropriate continuous flow of sample into a combustion tube carrier
stream, which directs the sample into the oxidation zone at a controlled and repeatable rate.
D7620 − 10 (2015)
FIG. 2 Typical Combustion Tube
7.8 Strip Chart Recorder—Equivalent electronic data logger, integrator or recorder (optional).
7.9 Balance—With a precision of 6 0.01 mg 60.01 mg (optional).
8. Reagents and Materials
8.1 Purity of Reagents—Reagent grade chemicals shall be used in tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
such specifications are available. Other grades may be used, provided, it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
8.2 Air—Filtered (with a 2 μm 2 μm filter).
8.3 Iso-octane, Toluene, Xylenes—Reagent grade. (Warning—Organic solvents are flammable.)
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For Suggestions on the testing of reagents not listed by
the American Chemical Society, see Annual Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
D7620 − 10 (2015)
TABLE 1 Typical Operating Conditions
Furnace Temperature 1050°C
Furnace Temperature 1050 °C
Furnace Pressure 28 kPa
Furnace Pressure 28 kPa
Photo Multiplier Tube (PMT) 40°C
Temperature
Photo Multiplier Tube (PMT) 40 °C
Temperature
PMT Voltage 900 V
Optics Purge Flow 80 to 100 mL/min
Optics Purge Flow 80 mL ⁄ min to 100 mL ⁄ min
8.4 Thiophene—FW84.14, Sulfur content 38.10 % (m/m).
8.4.1 Other sources of sulfur and diluent materials may be used if precision and accuracy are not degraded.
8.4.2 Apply the appropriate correction for chemical impurity.
8.5 Sulfur Stock Solution—1000 μg 1000 μg S/mL. Prepare a stock solution by accurately weighing 0.26240.2624 g 6 0.013
g 0.013 g of thiophene into a tared 100 mL 100 mL volumetric flask. Dilute to volume with selected solvent. This stock may be
further diluted to desired sulfur concentration.
8.5.1 Working standards should be reblended on a regular basis depending upon frequency of use and age.
NOTE 3—Typically, stock solutions have a useful life of about 3 months.
8.6 Quality Control (QC) Samples—Preferably, these are portions of one or more hydrocarbon materials that are stable and
representative of the samples of interest. These QC samples may be used to check the validity of the testing process as described
in Section 16.
9. Hazards
9.1 Consult current OSHA regulations, suppliers’ Material Safety Data Sheets, and local regulations for
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.