ASTM D6971-04
(Test Method)Standard Test Method for Measurement of Hindered Phenolic and Aromatic Amine Antioxidant Content in Non-zinc Turbine Oils by Linear Sweep Voltammetry
Standard Test Method for Measurement of Hindered Phenolic and Aromatic Amine Antioxidant Content in Non-zinc Turbine Oils by Linear Sweep Voltammetry
SCOPE
1.1 This test method covers the voltammetric determination of hindered phenol and aromatic amine antioxidants in new or used type non-zinc turbine oils in concentrations from 0.0075 mass % up to concentrations found in new oils by measuring the amount of current flow at a specified voltage in the produced voltammogram.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation:D6971–04
Standard Test Method for
Measurement of Hindered Phenolic and Aromatic Amine
Antioxidant Content in Non-zinc Turbine Oils by Linear
Sweep Voltammetry
This standard is issued under the fixed designation D 6971; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Summary of Test Method
1.1 This test method covers the voltammetric determination 3.1 A measured quantity of sample is dispensed into a vial
of hindered phenol and aromatic amine antioxidants in new or containing a measured quantity of acetone based electrolyte
used type non-zinc turbine oils in concentrations from 0.0075 solution and a layer of sand. When the vial is shaken, the
mass % up to concentrations found in new oils by measuring hindered phenol and aromatic amine antioxidants and other
the amount of current flow at a specified voltage in the solution soluble oil components present in the sample are
produced voltammogram. extracted into the solution and the remaining droplets sus-
1.2 This standard does not purport to address all of the pended in the solution are agglomerated by the sand. The
safety concerns, if any, associated with its use. It is the sand/droplet suspension is allowed to settle out and the
responsibility of the user of this standard to establish appro- hindered phenol and aromatic amine antioxidants dissolved in
priate safety and health practices and determine the applica- the solution are quantified by voltammetric analysis. The
bility of regulatory limitations prior to use. results are calculated and reported as mass % of antioxidant or
as millimoles (mmol) of antioxidant per litre of sample for
2. Referenced Documents
prepared and fresh oils and as a percent remaining antioxidant
2.1 ASTM Standards:
for used oils.
D 1193 Specification for Reagent Water 3.2 Voltammetric analysis is a technique that applies
D 2272 Test Method for Oxidation Stability of Steam Tur-
electro-analytic methods wherein a sample to be analyzed is
bine Engine Oils by Rotating Pressure Vessel mixed with an electrolyte and a solvent, and placed within an
D 4057 Practice for Manual Sampling of Petroleum and electrolytic cell. Data is obtained by measuring the current
Petroleum Products
passing through the cell as a function of the potential applied,
D 4378 Practice for In-Service Monitoring of Mineral Tur- and test results are based upon current, voltage, and time
bine Oils for Steam and Gas Turbines
relationships at the cell electrodes. The cell consists of a fluid
D 6224 Practice for In-Service Monitoring of Lubricating container into which is mounted a small, easily polarized,
Oil for Auxiliary Power Plant Equipment
working electrode, and a large, non-polarizable, reference
D 6810 Test Method for Measurement of Hindered Phe- electrode. The reference electrode should be massive relative
nolic Antioxidant Content In HL Turbine Oils by Linear
totheworkingelectrodesothatitsbehaviorremainsessentially
Sweep Voltammetry constant with the passage of small current; that is, it remains
2.2 ISO Standards:
unpolarized during the analysis period. Additional electrodes,
ISO 6743 Part 4, Lubricants, Industrial Oils, and Related such as auxiliary electrodes, can be added to the electrode
Products
system to eliminate the effects of resistive drop for high
resistancesolutions.Inperformingavoltammetricanalysis,the
potential across the electrodes is varied linearly with time, and
This test method is under the jurisdiction of ASTM Committee D02 on the resulting current is recorded as a function of the potential.
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
As the increasing voltage is applied to the prepared sample
D02.09.0C on Oxidation of Turbine Oils.
within the cell, the various additive species under investigation
Current edition approved July 1, 2004. Published July 2004.
withintheoilarecausedtoelectrochemicallyoxidize.Thedata
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
recorded during this oxidation reaction can then be used to
Standards volume information, refer to the standard’s Document Summary page on
determine the remaining useful life of the oil type. A typical
the ASTM website.
current-potential curve produced during the practice of the
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036. voltammetric test can be seen by reference to Fig. 1. Initially
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D6971–04
NOTE—x-axis = time (seconds) and y-axis is current (arbitrary units). Top line in Fig. 1 is voltammogram of a fresh R&O turbine oil showing valley
indicators before and after antioxidant valleys.
FIG. 1 Aromatic Amine and Hindered Phenol Voltammetric Response in the Neutral Test Solution with Blank Response Zeroed
the applied potential produces an electrochemical reaction 4.1.1 This test method is applicable to non-zinc type of
havingaratesoslowthatvirtuallynocurrentflowsthroughthe turbine oils as defined by ISO 6743 Part 4, Table 1. These are
cell. As the voltage is increased, as shown in Fig. 1, the refined mineral oils containing rust and oxidation inhibitors,
electro-active species (for example, substituted phenols) begin but not antiwear additives.
to oxidize at the working electrode surface, producing an 4.2 The test is also suitable for manufacturing control and
anodic rise in the current.As the potential is further increased, specification acceptance.
the decrease in the electro-active species concentration at the 4.3 When a voltammetric analysis is obtained for a turbine
electrode surface and the exponential increase of the oxidation oil inhibited with a typical synergistic mixture of hindered
rate lead to a maximum in the current-potential curve shown in phenol and aromatic amine antioxidants, there is an increase in
Fig. 1. the current of the produced voltammogram between 8 to 12 s
(or 0.8 to 1.2 V applied voltage) for the aromatic amines, and
4. Significance and Use
an increase in the current of the produced voltammogram
between 13 and 16 s (or 1.3 to 1.6 V applied voltage) for the
4.1 The quantitative determination of hindered phenol and
aromatic amine antioxidants in a new turbine oil measures the hindered phenols in the neutral acetone solution (Fig. 1: x-axis
1 s = 0.1 V). Hindered phenol antioxidants detected by
amount of these compounds that has been added to the oil as
voltammetric analysis include, but are not limited to, 2,6-di-
protection against oxidation. Beside phenols, turbine oils can
tert-butyl-4-methylphenol; 2,6-di-tert-butylphenol; and 4,4’-
be formulated with other antioxidants such as amines which
Methylenebis (2,6-di-tert-butylphenol). Aromatic amine anti-
can extend the oil life. In used oil, the determination measures
the amount of original (hindered phenol and aromatic amine) oxidants detected by voltammetric analysis include, but are not
limited to, phenyl alpha naphthylamines, and alkylated diphe-
antioxidants remaining after oxidation has reduced its initial
concentration. This test method is not designed or intended to nylamines.
4.4 For turbine oil containing only aromatic amines as
detect all of the antioxidant intermediates formed during the
thermal and oxidative stressing of the oils, which are recog- antioxidants, there will only be an increase in the current of the
produced voltammogram between 8 to 12 seconds (or 0.8 to
nized as having some contribution to the remaining useful life
of the used or in-service oil. Nor does it measure the overall 1.2 V applied voltage) for the aromatic amines, by using the
neutral acetone test solution (first peak in Fig. 1).
stabilityofanoil,whichisdeterminedbythetotalcontribution
of all species present. Before making final judgment on the 4.5 For turbine oils containing only hindered phenolic
antioxidants, it is preferable to use a basic alcohol solution
remaining useful life of the used oil, which might result in the
replacement of the oil reservoir, it is advised to perform
additional analytical techniques (as in accordance with Test
Methods D 6224 and D 4378; see also Test Method D 2272),
Voltages listed with respect to reference electrode. The voltammograms shown
having the capability of measuring remaining oxidative life of
in Figs. 1 and 2 were obtained with a platinum reference electrode and a voltage
the used oil. scan rate of 0.1 V/s.
D6971–04
NOTE—x-axis = time (seconds) and y-axis is current (arbitrary units) with top line in Fig. 2 showing the fresh oil.
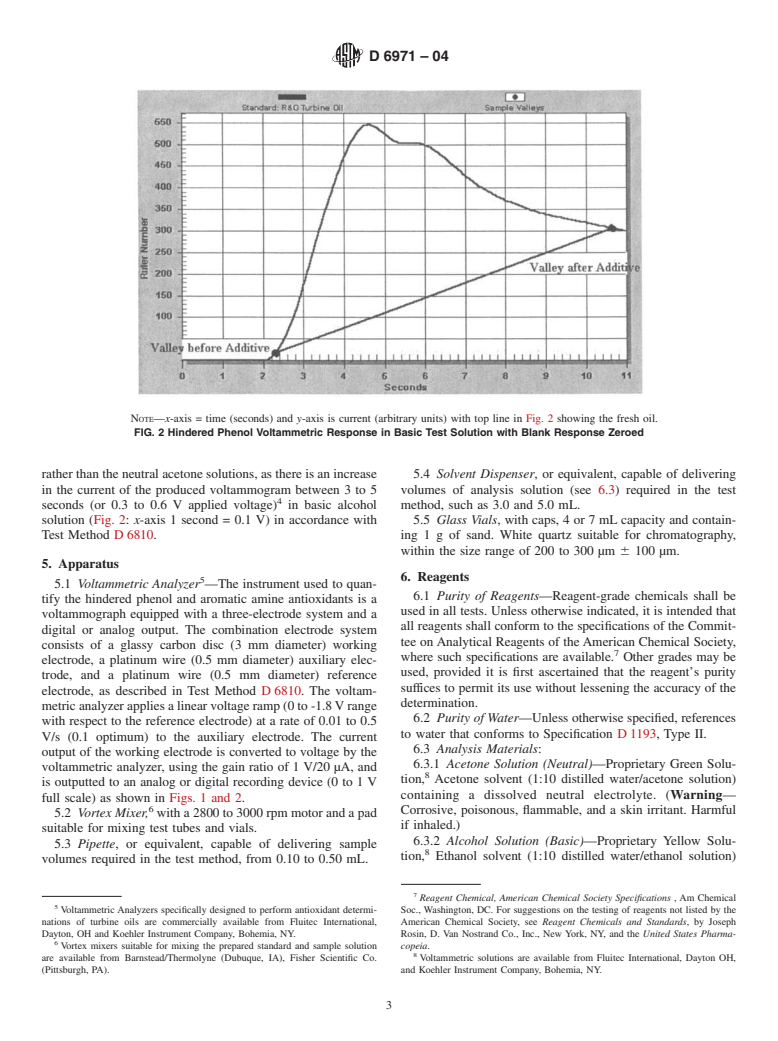
FIG. 2 Hindered Phenol Voltammetric Response in Basic Test Solution with Blank Response Zeroed
rather than the neutral acetone solutions, as there is an increase 5.4 Solvent Dispenser, or equivalent, capable of delivering
in the current of the produced voltammogram between 3 to 5 volumes of analysis solution (see 6.3) required in the test
seconds (or 0.3 to 0.6 V applied voltage) in basic alcohol method, such as 3.0 and 5.0 mL.
solution (Fig. 2: x-axis 1 second = 0.1 V) in accordance with 5.5 Glass Vials, with caps, 4 or 7 mL capacity and contain-
Test Method D 6810. ing1gof sand. White quartz suitable for chromatography,
within the size range of 200 to 300 µm 6 100 µm.
5. Apparatus
6. Reagents
5.1 Voltammetric Analyzer —The instrument used to quan-
6.1 Purity of Reagents—Reagent-grade chemicals shall be
tify the hindered phenol and aromatic amine antioxidants is a
used in all tests. Unless otherwise indicated, it is intended that
voltammograph equipped with a three-electrode system and a
all reagents shall conform to the specifications of the Commit-
digital or analog output. The combination electrode system
tee onAnalytical Reagents of theAmerican Chemical Society,
consists of a glassy carbon disc (3 mm diameter) working
where such specifications are available. Other grades may be
electrode, a platinum wire (0.5 mm diameter) auxiliary elec-
used, provided it is first ascertained that the reagent’s purity
trode, and a platinum wire (0.5 mm diameter) reference
suffices to permit its use without lessening the accuracy of the
electrode, as described in Test Method D 6810. The voltam-
determination.
metricanalyzerappliesalinearvoltageramp(0to-1.8Vrange
6.2 Purity of Water—Unless otherwise specified, references
with respect to the reference electrode) at a rate of 0.01 to 0.5
to water that conforms to Specification D 1193, Type II.
V/s (0.1 optimum) to the auxiliary electrode. The current
6.3 Analysis Materials:
output of the working electrode is converted to voltage by the
6.3.1 Acetone Solution (Neutral)—Proprietary Green Solu-
voltammetric analyzer, using the gain ratio of 1 V/20 µA, and
tion, Acetone solvent (1:10 distilled water/acetone solution)
is outputted to an analog or digital recording device (0 to 1 V
containing a dissolved neutral electrolyte. (Warning—
full scale) as shown in Figs. 1 and 2.
Corrosive, poisonous, flammable, and a skin irritant. Harmful
5.2 Vortex Mixer, with a 2800 to 3000 rpm motor and a pad
if inhaled.)
suitable for mixing test tubes and vials.
6.3.2 Alcohol Solution (Basic)—Proprietary Yellow Solu-
5.3 Pipette, or equivalent, capable of delivering sample
tion, Ethanol solvent (1:10 distilled water/ethanol solution)
volumes required in the test method, from 0.10 to 0.50 mL.
Reagent Chemical, American Chemical Society Specifications , Am Chemical
Voltammetric Analyzers specifically designed to perform antioxidant determi- Soc., Washington, DC. For suggestions on the testing of reagents not listed by the
nations of turbine oils are commercially available from Fluitec International, American Chemical Society, see Reagent Chemicals and Standards, by Joseph
Dayton, OH and Koehler Instrument Company, Bohemia, NY. Rosin, D. Van Nostrand Co., Inc., New York, NY, and the United States Pharma-
Vortex mixers suitable for mixing the prepared standard and sample solution copeia.
are available from Barnstead/Thermolyne (Dubuque, IA), Fisher Scientific Co. Voltammetric solutions are available from Fluitec International, Dayton OH,
(Pittsburgh, PA). and Koehler Instrument Company, Bohemia, NY.
D6971–04
containingadissolvedbaseelectrolyte.(Warning—Corrosive, 8.3 Voltammetric Reading—After the operator has selected
poisonous, flammable, and a skin irritant. Harmful if inhaled.) the valleys before and after the antioxidant peaks (as shown in
Fig. 1), the software (R-DMS ) will automatically identify and
6.3.3 Alcohol Cleansing Pads—70 % isopropyl alcohol
calculate the area above the baseline between the two valley
saturatedcleansingpads(alcoholpreparedskincleansingpads,
indicators. This calculated area is then used for the sample
for the preparation of the skin prior to injection (antiseptic)).
reading (used oil), which will be established by comparing the
used oil area to its standard (see Fig. 3) and make remaining
7. Sampling
antioxidant calculations (see Section 9).
7.1 Obtain the sample in accordance with Practice D 4057. 8.4 Calibration (Blank Reading) Procedure—Pipette 5.0
mL of analysis solution intoa7mL vial or other suitable
container containing1gof sand. Insert the electrode of the
8. Procedure
voltammetric analyzer into the analysis solution to wet the
8.1 The voltammetric analyzer used in this test method
bottom surface of the electrode, remove, and rub dry the
gives linear results between 2 to 50 mmol for hindered phenols
bottom electrode surface with a lint free paper towel. Insert the
and aromatic amines using an oil sample size of 0.40 mL and
electrode into the vial so that the bottom of the electrode is
5.0 mL of the analysis solvent. The corresponding range of
submerged in the analysis solution without resting on the sand
mass % depends on the molecular weight of the hindered
layeronthebottomofthevial.Placethevial/probeuprightinto
phenol and aromatic amine, and the density of the base oil. For
rack or foam block for testing. Perform the voltammetric
instance, the mass % range of 0.044 to 1.1 is equal to 2 to 50
analysis (see 5.1). Record the voltammetric reading in the
mmol/L for a hindered phenol containing one hydroxyl group
voltage range of aromatic amines, 0.8 to 1.1 V and the
and with a molecular weight of 220 g/mol (2,6-di-tert-butyl-
phenols, 1.3 to 1.6 V
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.