ASTM B798-95(2009)
(Test Method)Standard Test Method for Porosity in Gold or Palladium Coatings on Metal Substrates by Gel-Bulk Electrography
Standard Test Method for Porosity in Gold or Palladium Coatings on Metal Substrates by Gel-Bulk Electrography
SIGNIFICANCE AND USE
Noble metal coatings, particularly gold or palladium, are often specified for the contacts of separable electrical connectors and other devices. Electrodeposits are the form of gold or palladium which is most used on contacts, although gold and palladium are also employed as clad metal and as weldments on the contact surface. The intrinsic nobility of gold and to a certain extent palladium enables them to resist the formation of insulating films that could interfere with reliable contact operation.
In order that the nobility of gold be assured, porosity, cracks, and other defects in the coating that expose base metal substrates and underplates must be minimal or absent, except in those cases where it is feasible to use the contacts in structures that shield the surface from the environment or where corrosion inhibiting surface treatments for the deposit are employed. The level of porosity in the coating that may be tolerable depends on the severity of the environment to the underplate or substrate, design factors for the contact device like the force with which it is mated, circuit parameters, and the reliability of contact operation that it is necessary to maintain. Also, when present, the location of pores on the surface is important. If the pores are few in number or are outside of the zone of contact of the mating surfaces, their presence can often be tolerated.
Methods for determining pores on a contact surface are most suitable if they enable their precise location and numbers to be determined. Contact surfaces are often curved or irregular in shape, and testing methods should be suitable for them. In addition, the severity of porosity-determining tests may vary from procedures capable of detecting all porosity to procedures that detect only gross defects. The test method in this document is generally regarded as severe.
The relationship of porosity levels revealed by particular tests to contact behavior must be made by the user of these tests through pr...
SCOPE
1.1 This test method covers equipment and techniques for determining porosity in noble metal coatings, particularly electrodeposits and clad metals used on electrical contacts.
1.2 The test method is designed to show whether the porosity level is less or greater than some value which by experience is considered by the user to be acceptable for the intended application.
1.3 Other porosity testing methods are outlined in Guide B 765. Detailed critical reviews of porosity testing are also available. Other porosity test methods are B 735, B 741, B 799, and B 809.
1.4 The values stated in SI units are to be regarded as standard. The values in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to become familiar with all hazards including those identified in the appropriate Material Safety Data Sheet (MSDS) for this product/material as provided by the manufacturer, to establish appropriate safety and health practices, and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Sections 7 and 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: B798 − 95(Reapproved 2009)
Standard Test Method for
Porosity in Gold or Palladium Coatings on Metal Substrates
by Gel-Bulk Electrography
This standard is issued under the fixed designation B798; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope B735Test Method for Porosity in Gold Coatings on Metal
Substrates by Nitric Acid Vapor
1.1 This test method covers equipment and techniques for
B741Test Method for Porosity In Gold Coatings On Metal
determining porosity in noble metal coatings, particularly
Substrates By Paper Electrography (Withdrawn 2005)
electrodeposits and clad metals used on electrical contacts.
B765GuideforSelectionofPorosityandGrossDefectTests
1.2 The test method is designed to show whether the
for Electrodeposits and Related Metallic Coatings
porosity level is less or greater than some value which by
B799Test Method for Porosity in Gold and Palladium
experience is considered by the user to be acceptable for the
Coatings by Sulfurous Acid/Sulfur-Dioxide Vapor
intended application.
B809Test Method for Porosity in Metallic Coatings by
Humid Sulfur Vapor (“Flowers-of-Sulfur”)
1.3 Other porosity testing methods are outlined in Guide
B765. Detailed critical reviews of porosity testing are also
3. Terminology
available. Other porosity test methods are B735, B741, B799,
3.1 Definitions—Many terms used in this test method are
and B809.
defined in Terminology B542 and terms relating to metallic
1.4 The values stated in SI units are to be regarded as
coatings are defined in Terminology B374.
standard. The values in parentheses are for information only.
3.2 Definitions of Terms Specific to This Standard:
1.5 This standard does not purport to address all of the
3.2.1 decorations, n—those reaction products emanating
safety concerns, if any, associated with its use. It is the
from the pores that provide visual contrast with the gel
responsibility of the user of this standard to become familiar
medium.
with all hazards including those identified in the appropriate
3.2.2 measurement area (or “significant surface”), n—the
Material Safety Data Sheet (MSDS) for this product/material
surface that is examined for the presence of porosity. The
as provided by the manufacturer, to establish appropriate
significant surfaces or measurement areas of the part to be
safety and health practices, and determine the applicability of
tested shall be indicated on the drawing of the part or by
regulatory limitations prior to use. For specific hazard
provision of suitably marked samples.
statements, see Sections 7 and 8.
3.2.2.1 Discussion—For specification purposes, the signifi-
2. Referenced Documents
cant surfaces or measurement areas are often defined as those
portionsofthesurfacethatareessentialtotheserviceabilityor
2.1 ASTM Standards:
functionofthepart,suchasitscontactproperties,orwhichcan
B374Terminology Relating to Electroplating
be the source of corrosion products or tarnish films that
B542Terminology Relating to Electrical Contacts andTheir
interfere with the function of the part.
Use
3.2.3 metallic coatings, n—include platings, claddings, or
other metallic layers applied to the substrate. The coatings can
This test method is under the jurisdiction of ASTM Committee B02 on
Nonferrous Metals and Alloys and is the direct responsibility of Subcommittee
comprise a single metallic layer or a combination of metallic
B02.11 on Electrical Contact Test Methods.
layers.
Current edition approved Oct. 1, 2009. Published October 2009. Originally
approved in 1988. Last previous edition approved in 2005 as B798–95(2005).
3.2.4 porosity, n—the presence of any discontinuity, crack,
DOI: 10.1520/B0798-95R09.
orholeinthecoatingthatexposesadifferentunderlyingmetal.
Nobel, F. J., Ostrow, B. D., and Thompson, D. W., “Porosity Testing of Gold
3.2.5 underplate, n—a metallic coating layer between the
Deposity,” Plating,Vol 52, 1965, p. 1001, and Krumbein S. J., “PorosityTesting of
Contact Platings,” Proceedings, Connectors and Interconnection Technology
substrate and the topmost layer or layers. The thickness of an
Symposium, October 1987, p. 47.
underplate is usually greater that 0.8 µm (30 µin.).
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
B798 − 95 (2009)
4. Summary of Test Method 5.4 Therelationshipofporositylevelsrevealedbyparticular
teststocontactbehaviormustbemadebytheuserofthesetests
4.1 This test method is an electrographic technique, “gel-
through practical experience or judgment. Thus, absence of
bulk electrography.” The specimen is made the anode in a cell
porosity in the coating may be a requirement for some
containing a solid or semisolid electrolyte of gelatin, conduct-
applications, while a few pores in the contact zone may be
ing salts, and an indicator. Application of current to this cell
acceptable for others.
results in the migration of base medal ions through continuous
pores. Reaction of cations with an indicator gives rise to
5.5 This test method is capable of detecting porosity or
colored reaction products at pore sites which may be counted
other defects in gold or palladium coatings that could partici-
through the clear gel. Individual spots are counted with the aid
pateinsubstratecorrosionreactions.Inaddition,itcanbeused
of a loupe or low power stereomicroscope.
on contacts having complex geometry such as pin-socket
contacts (although difficulty may be experienced in inspecting
4.2 This test method is suitable for coatings containing
deep recesses).
75% or more of gold on substrates of silver, nickel, copper,
and its alloys, which are commonly used in electrical contacts.
This test method is also suitable for coatings of 95% or more 6. Limitations
of palladium on nickel, copper and its alloys.
6.1 This test is considered destructive in that it reveals the
4.3 These porosity tests involve corrosion reactions in presence of porosity by contaminating the surface with corro-
which the products delineate defect sites in coatings. Since the
sionproductsandbyunder-cuttingthecorrodiblemetalatpore
chemistry and properties of these products do not resemble
sites and at unplated areas. In addition, the surface is coated
those found in natural or service environments, these tests are
with a corrosive gel mixture which is difficult to remove
not recommended for prediction of the electrical performance
completely. Any parts exposed to the gel test shall not be
of contacts unless correlation is first established with service
placed in service.
experience.
6.2 Thegel-bulkprocedureisnotassensitivetosmallpores
and is more complex than porosity tests involving gaseous
5. Significance and Use
corrodants (see Test Methods B735 and B799). It also
5.1 Noblemetalcoatings,particularlygoldorpalladium,are
involves more chemicals, preparation, and auxiliary equip-
often specified for the contacts of separable electrical connec-
ment.
tors and other devices. Electrodeposits are the form of gold or
6.3 This test is intended to be used for quantitative descrip-
palladium which is most used on contacts, although gold and
tions of porosity (such as number of pores per unit area or per
palladium are also employed as clad metal and as weldments
contact) only on measurement areas where coatings have pore
on the contact surface. The intrinsic nobility of gold and to a
certainextentpalladiumenablesthemtoresisttheformationof densities that are sufficiently low so that the corrosion sites are
insulating films that could interfere with reliable contact well separated and can be readily resolved. As a general
operation. guideline this can be achieved for pore densities up to about
25/cm .
5.2 In order that the nobility of gold be assured, porosity,
cracks, and other defects in the coating that expose base metal
6.4 For this purpose, the measurement area, or “significant
substrates and underplates must be minimal or absent, except
surface,” shall be defined as those portions of the surface that
in those cases where it is feasible to use the contacts in
are essential to the serviceability or function of the part, such
structures that shield the surface from the environment or
as its contact properties, or which can be the source of
where corrosion inhibiting surface treatments for the deposit
corrosion products or tarnish films that interfere with the
are employed. The level of porosity in the coating that may be
function of the part. When necessary, the significant surfaces
tolerable depends on the severity of the environment to the
shall be indicated on the drawings of the parts, or by the
underplate or substrate, design factors for the contact device
provision of suitably marked samples.
liketheforcewithwhichitismated,circuitparameters,andthe
6.5 The test applicability to platings of varying thickness is
reliability of contact operation that it is necessary to maintain.
a function of the quality of the plating.
Also, when present, the location of pores on the surface is
important. If the pores are few in number or are outside of the
6.6 The applicability of this test method to localized plat-
zoneofcontactofthematingsurfaces,theirpresencecanoften
ings or claddings with adjacent exposed substrate is limited by
be tolerated.
the efficacy of coatings applied to mask the non-noble areas to
5.3 Methods for determining pores on a contact surface are prevent gross decoration of the surfaces under test. Users of
most suitable if they enable their precise location and numbers
this method are required to develop their own techniques for
tobedetermined.Contactsurfacesareoftencurvedorirregular masking such exposed substrate areas.
in shape, and testing methods should be suitable for them. In
addition, the severity of porosity-determining tests may vary
fromprocedurescapableofdetectingallporositytoprocedures
For example, Clarke, M., “Porosity and Porosity Tests,” in “Properties of
thatdetectonlygrossdefects.Thetestmethodinthisdocument
Electrodeposits,” edited by Sard, Leidheiser, and Ogburn, The Electrochemical
is generally regarded as severe. Society, 1975, p. 122.
B798 − 95 (2009)
7. Apparatus
7.1 Test Vessel may be made of glass, acrylic resin, or other
inert uncolored transparent material. It shall have thin-walled
flat sides, and be of a size appropriate to the sample to be
tested.
7.2 Power Supply, 0to1 A and0to10 V dc, an
electronically-regulated, constant-current (65%) apparatus is
preferred.
7.3 dc Milliammeter and Separate dc Voltmeter.
7.4 Cathode Material in the form of foil or wire made of
platinum or gold is required. The cathode and specimen
(anode) areas shall be approximately the same. Additionally,
goldorplatinumwireforcathodeandanodeareneededforthat
portionofthehook-upthatisinthereagentsolution.Itmaybe
convenient to use small alligator clips to secure the lead wires
to the cathode and anode. These clips must be heavily gold
plated so as to be entirely free of porosity. A variation of this
procedure, suitable for samples having relatively few pores, is
to use a second identical test sample as the cathode. The test
can be run with current first in the forward, then in the reverse
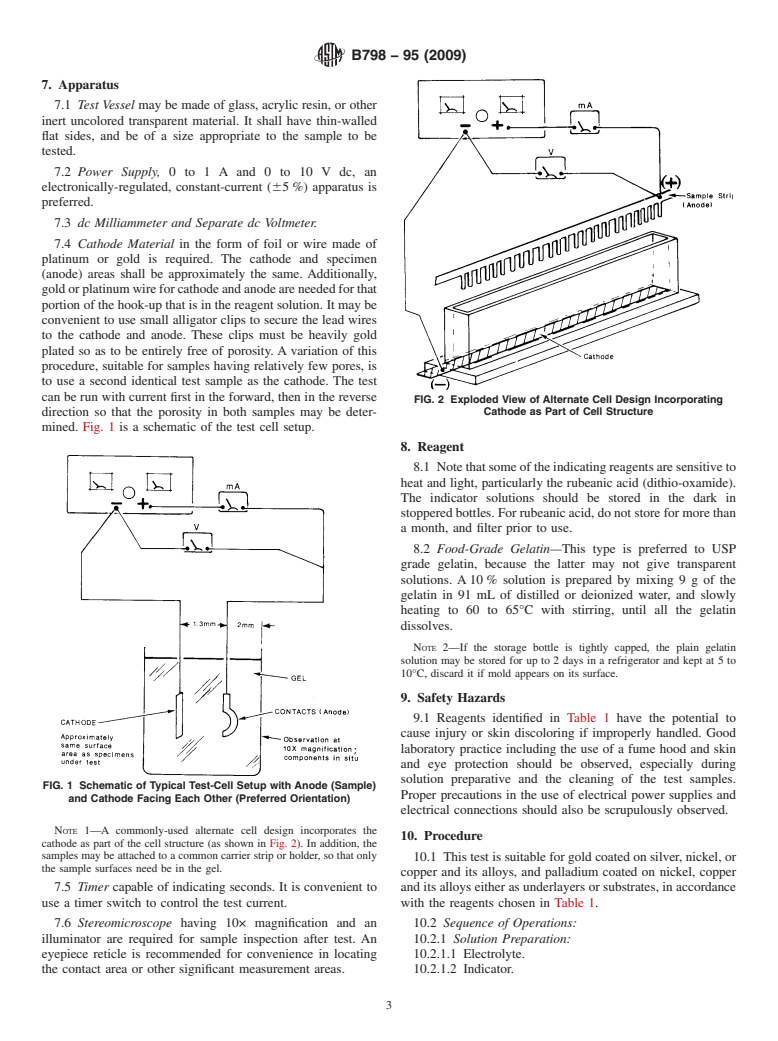
FIG. 2 Exploded View of Alternate Cell Design Incorporating
direction so that the porosity in both samples may be deter- Cathode as Part of Cell Structure
mined. Fig. 1 is a schematic of the test cell setup.
8. Reagent
8.1 Notethatsomeoftheindicatingreagentsaresensitiveto
heat and light, particularly the rubeanic acid (dithio-oxamide).
The indicator solutions should be stored in the dark in
stopperedbottles.Forrubeanicacid,donotstoreformorethan
a month, and filter prior to use.
8.2 Food-Grade Gelatin—This type is preferred to USP
grade gelatin, because the latter may not give transparent
solutions. A10% solution is prepared by mixing9gofthe
gelatin in 91 mL of distilled or deionized water, and slowly
heating to 60 to 65°C with stirring, until all the gelatin
dissolves.
NOTE 2—If the storage bottle is tightly capped, the plain gelatin
solution may be stored for up to 2 days in a refrigerator and kept at 5 to
10°C, discard it if mold appears on its surface.
9. Safety Hazards
9.1 Reagents identified in Table 1 have the potential to
cause injury or skin discoloring if improperly handled. Good
laboratory practice including the use of a fume hood and skin
and eye protection should be observed, especially during
solution preparative and the cleaning of the test samples.
FIG. 1 Schematic of Typical Test-Cell Setup with Anode (Sample)
Proper precautions in the use of electrical power supplies and
and Cathode Facing Each Other (Preferred Orientation)
electrical connections should also be scrupulously observed.
NOTE 1—A commonly-used alternate cell design incorporates the
10. Procedure
cathode as part of the cell structure (as shown in Fig. 2). In addition, the
samples may be attached to a common carrier strip or holder, so that only
10.1 Thistestissuitableforgoldcoatedonsilver,nickel,or
the sample surfaces need be in the gel.
copper and its alloys, and palladium coated on nickel, copper
7.5 Timer capable of indicating seconds. It is convenient to anditsalloyseitherasunderlayersorsubstrates,inaccordance
use a timer switch to control the test current. with the reagents chosen in Table 1.
7.6 Stereomicroscope having 10× magnification and an 10.2 Sequence of Operations:
illuminator are required for sample inspection after test. An 10.2.1 Solution Preparation:
eyepiece reticle is recommended for convenience in locating 10.2.1.1 Electrolyte.
the contact area or other significant measurement areas. 10.2.1.2 Indicator.
B798 − 95 (2009)
TABLE 1 Guide to Gel Porosity Testing Solutions
Test for Electrolyte (Aqueous) Indicator Indicating Color Comments
A
Copper 4 % sodium carbonate+1%so- saturated solution of rubeanic dark olive green also detects nickel, cobalt
dium nitrate acid in ethanol
Copper 4 % sodium carbonate+1%so- 7.5 % potassium ferrocyanide in brown -------
dium nitrate water
A
Nickel 4 % sodium carbonate+1%so- saturated solution of rubeanic blue-blue violet also detects copper, cobalt
dium nitrate acid
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.