ASTM C1108-99
(Test Method)Standard Test Method for Plutonium by Controlled-Potential Coulometry
Standard Test Method for Plutonium by Controlled-Potential Coulometry
SCOPE
1.1 This test method describes the determination of plutonium in solutions of unirradiated nuclear-grade (that is, high-purity) materials by controlled-potential coulometry. Controlled-potential coulometry may be performed in a choice of supporting electrolytes, such as 0.9 HNO 3 , 1 HClO 4 , 1 HCl, 5 HCl, and 0.5 H 2 SO 4 . Limitations on the use of selected supporting electrolytes are discussed in Section 5. Optimum quantities of plutonium for this procedure are 5 to 10 mg.
1.2 Plutonium-bearing materials are radioactive and toxic. Adequate laboratory facilities, such as gloved boxes, fume hoods, controlled ventilation, etc., along with safe techniques must be used in handling specimens containing these materials.
1.3 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: C 1108 – 99
Standard Test Method for
Plutonium by Controlled-Potential Coulometry
This standard is issued under the fixed designation C1108; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope C1168 Practice for Preparation and Dissolution of Pluto-
nium Materials for Analysis
1.1 This test method describes the determination of pluto-
C1210 Guide for Establishing a Measurement System
nium in solutions of unirradiated nuclear-grade (that is, high-
Quality Control Program for Analytical Chemistry Labo-
purity) materials by controlled-potential coulometry.
ratories Within the Nuclear Industry
Controlled-potential coulometry may be performed in a choice
C1297 Guide for Qualification of Laboratory Analysts for
of supporting electrolytes, such as 0.9 M HNO,1 M HClO,1
3 4
the Analysis of Nuclear Fuel Cycle Materials
M HCl, 5 M HCl, and 0.5 M H SO . Limitations on the use of
2 4
E691 Practice for Conducting an Interlaboratory Study to
selected supporting electrolytes are discussed in Section 5.
Determine the Precision of a Test Method
Optimumquantitiesofplutoniumforthisprocedureare5to10
mg.
3. Summary of Test Method
1.2 Plutonium-bearing materials are radioactive and toxic.
3.1 In a controlled-potential coulometric measurement, the
Adequate laboratory facilities, such as gloved boxes, fume
substancebeingdeterminedreactsatanelectrode,thepotential
hoods, controlled ventilation, etc., along with safe techniques
of which is maintained at such a value that unwanted electrode
mustbeusedinhandlingspecimenscontainingthesematerials.
reactions are precluded under the prevailing experimental
1.3 The values stated in SI units are to be regarded as the
conditions. Those substances which have reduction-oxidation
standard. The values given in parentheses are for information
(redox) potentials near that of the ion being determined
only.
constitute interferences. Electrolysis current decreases expo-
1.4 This standard does not purport to address all of the
nentially as the reaction proceeds, until constant background
safety concerns, if any, associated with its use. It is the
current is obtained. Detailed discussions of the theory and
responsibility of the user of this standard to establish appro-
applications of this technique have been published (1, 2, 3, 4,
priate safety and health practices and determine the applica-
5, 6). The control-potential adjustment technique (7) can be
bility of regulatory limitations prior to use.
used to terminate the electrolysis of the specimen at constant
2. Referenced Documents background current without exhaustive electrolysis with con-
siderable reduction in operating time. Use of the control-
2.1 ASTM Standards:
potential adjustment technique requires that the coulometer
C1009 Guide for Establishing a Quality Assurance Pro-
integrator be capable of operations in a bipolar mode and that
gram for Analytical Chemistry Laboratories Within the
2 the plutonium-containing solution be of high purity, that is,
Nuclear Industry
nuclear grade.
C1068 Guide for Qualification of Measurement Methods
3 3.2 Plutonium(IV) is reduced to Pu(III) at a working elec-
by a Laboratory Within the Nuclear Industry
trode maintained at a potential more negative than the formal
C1128 Guide for Preparation of Working Reference Mate-
redox potential. Plutonium(III) is oxidized to Pu(IV) at a
rials for Use in the Analysis of Nuclear fuel Cycle
potential more positive than the formal redox potential. The
Materials
quantity of plutonium electrolyzed is calculated from the net
C1156 Guide for Establishing Calibration for a Measure-
number of coulombs required for the electrolysis, according to
ment Method Used to Analyze Nuclear Fuel Cycle Mate-
Faraday’s law. Corrections for incomplete reaction, derived
rials
from the Nernst equation, must be applied for electrolysis of
the sample aliquot (7, 8).
~Q 2 Q ! M
ThistestmethodisunderthejurisdictionofASTMCommitteeC-26onNuclear s b
W 5 (1)
Fuel Cycle and is the direct responsibility of Subcommittee C26.05 on Methods of nFf
Test.
Current edition approved Jan. 10, 1999. Published February 1999. Originally
published as C1108–88. Last previous edition C1108–93.
2 4
A Julie 100-V precision resistor number NB102A, accurate to 0.0015%, has Annual Book of ASTM Standards, Vol 14.02.
been found satisfactory. Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
Annual Book of ASTM Standards, Vol 12.01. this test method.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
C 1108
direct interference. Iron must be removed by prior separation,
where:
or the effect of its presence must be corrected by a separate
W 5 grams of plutonium,
measurement of the iron concentration in the sample solution.
Q 5 coulombs required by the electrolysis,
s
Q 5 coulombs of background current, In 1 M HCl, 1 M HNO,or1 M HClO , iron interferes to a
b 3 4
M 5 gram-atomic weight of plutonium (must be adjusted
much lesser extent. The effect of iron in these supporting
for isotopic composition),
electrolytes may be minimized by the choice of redox poten-
n 5 numberofelectronsinvolvedintheelectrodereaction
tials, by a secondary titration (10), or by electrochemical
(for Pu(III)→ Pu(IV), n 51),
correction (12, 13).
F 5 Faraday constant, coulombs/equivalent, and
5.5 Nitrites—Nitrites are electrochemically active; there-
f 5 fraction of plutonium electrolyzed.
fore, saturated sulfamic acid solution should be added to the
electrolyte in the cell to destroy any interfering nitrites.
4. Significance and Use
5.6 Sulfate—Becauseofthecomplexingactionofsulfateon
4.1 Factors governing selection of a method for the deter-
Pu(IV) and the resultant shift in the redox potential of the
mination of plutonium include available quantity of sample,
Pu(III)-Pu(IV) couple, only small amounts of sulfate are
sample purity, desired level of reliability, and equipment.
tolerable in HNO , HCl, and HClO electrolytes. When using
3 4
4.1.1 This test method determines 5 to 10 mg of plutonium
these supporting electrolytes, specimens should be fumed to
with prior dissolution using Practice C1168.
dryness to assure adequate removal of excess sulfate (see
4.1.2 This test method calculates plutonium assay using
10.1.3).
physical constants as reference standards.
NOTE 1—Interference from a sulfate concentration of >0.004 M in 1 M
4.1.3 Chemical standards are used for quality control when
HClO has been reported (10).
prior chemical separation of plutonium is necessary to remove
5.7 Fluoride—Freefluoridecannotbetoleratedandmustbe
interferences (9).
removed from the specimen. Evaporation of the specimen in
4.2 Committee C-26 Safeguards Statement :
HNO toalowvolumeandfumingwithH SO areeffectivein
4.2.1 The materials (plutonium metal, plutonium oxide or 3 2 4
removing fluoride.
mixed oxide [(U, Pu) O ] powders and pellets) to which this
5.8 Oxygen—In HNO , HCl, and HClO supporting elec-
test method applies are subject to nuclear safeguards regula- 3 4
trolytes, oxygen may be an interference. In H SO , oxygen
2 4
tions governing their possession and use. Materials for use by
does interfere and must be removed. Purging the specimen
the commercial nuclear community must also meet composi-
with high-purity argon prior to and during the coulometric
tional specifications.
determination is recommended for all electrolytes.
4.2.2 The analytical method in this test method both meets
U. S. Department of Energy guidelines for acceptability of a
6. Apparatus
measurementmethodforgenerationofsafeguardsaccountabil-
6.1 Controlled-Potential Coulometer—A coulometer with
ity measurement data and also provides data that may be used
to demonstrate specification compliance in buyer-seller inter- the following specifications is recommended to achieve highly
precise and accurate results. (Room temperature stability of
actions.
61°C is recommended to ensure optimum instrument perfor-
5. Interferences
mance. Instruments with smaller output current or smaller
voltage span may be satisfactory.)
5.1 Interferenceiscausedbyionsthatareelectrochemically
Potentiostat (6)
active in the range of redox potentials used or by species that
Output voltage >25 V
prevent attainment of 100% current efficiency (for example,
Output current >200 mA
reductants, oxidants, and organic matter).
Open-loop response d-c gain >10
Unity-gain bandwidth >300 kHz
5.2 Polymer—Polymerized plutonium is not electrochemi-
Full-power response >10 kHz (slewing rate 0.5 V/µs)
cally active (10) and thus is neither reduced nor oxidized. The
Voltage zero offset stability >1-mV long term
presence of polymerized plutonium will give low results. The
Input d-c resistance >50 MV
polymer may be converted to electrochemically active species Input d-c current <50 nA
d-c control voltage span 64V
by HF treatment (10).
Resolution, hum, and drift <1 mV
5.3 Pu(VI)—Plutonium(VI) is only partially reduced to
Stability through extreme of line and 65mV
load variation
Pu(III) in 1 M HNO , HCl, or HClO supporting electrolyte
3 4
Digital Integrator (14)
solutions; therefore, the presence of Pu(VI) can lead to
Nonlinearity of V/F converter <0.01 % full scale
inaccurate results when present even as a small fraction of the
Full scale error adjustable to zero
Input offset voltage error adjustable to zero
totalplutonium.Plutonium(VI)iscompletelyreducedin0.5 M
Output readability <1 µg Pu
H SO (10) or 5.5 M HCl (11) supporting electrolyte.
2 4
Integrating capacity >10 C
5.4 Iron—In 0.5 M H SO supporting electrolyte, iron is
2 4 Accuracy <0.01 %
reduced and oxidized at essentially the same formal redox
6.2 Digital Voltmeter, 15-V range, 5 ⁄2digits accurate to
potentials as the Pu(III)-Pu(IV) couple and thus constitutes a
10 7
0.01% of full scale on all ranges. Input resistance >10 V.
Based upon Committee C-26 Safeguards Matrix (C1009, C1068, C1128,
C1156, C1210, and C1297). AHewlett-Packard3455ADVMhasbeenfoundtoexceedthesespecifications.
C 1108
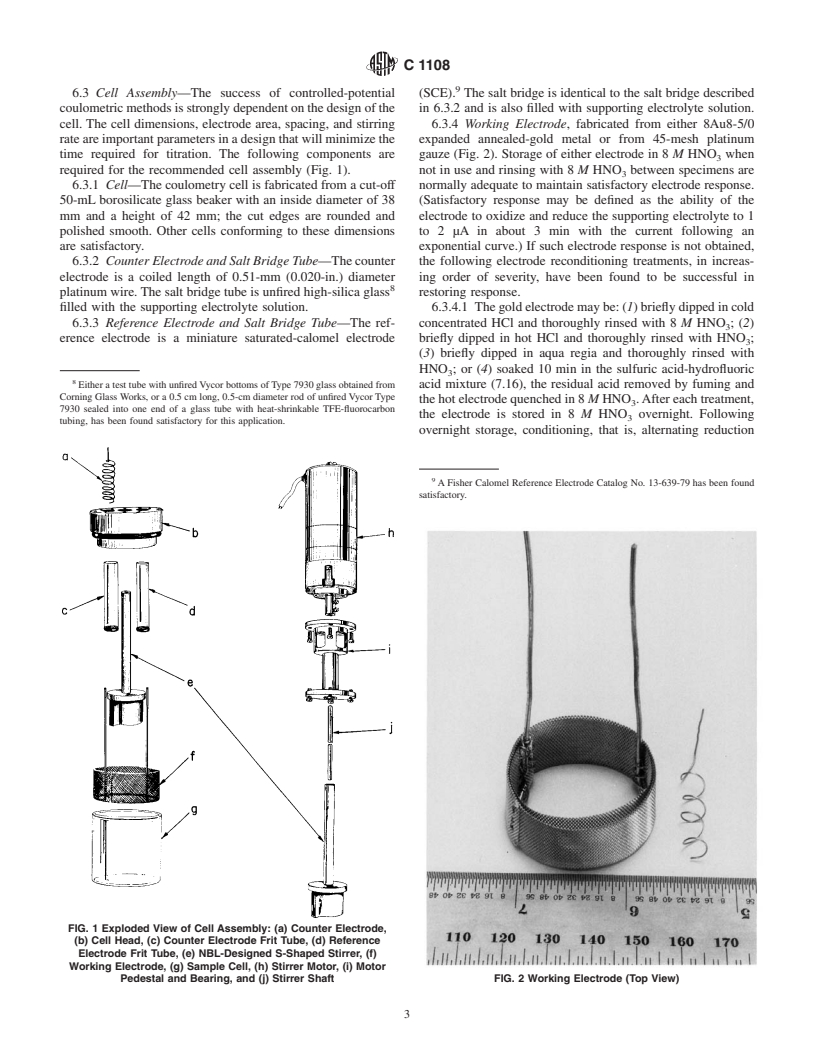
6.3 Cell Assembly—The success of controlled-potential (SCE). The salt bridge is identical to the salt bridge described
coulometricmethodsisstronglydependentonthedesignofthe in 6.3.2 and is also filled with supporting electrolyte solution.
cell. The cell dimensions, electrode area, spacing, and stirring 6.3.4 Working Electrode, fabricated from either 8Au8-5/0
rateareimportantparametersinadesignthatwillminimizethe expanded annealed-gold metal or from 45-mesh platinum
time required for titration. The following components are gauze (Fig. 2). Storage of either electrode in 8 M HNO when
required for the recommended cell assembly (Fig. 1). not in use and rinsing with 8 M HNO between specimens are
6.3.1 Cell—The coulometry cell is fabricated from a cut-off normally adequate to maintain satisfactory electrode response.
50-mL borosilicate glass beaker with an inside diameter of 38 (Satisfactory response may be defined as the ability of the
mm and a height of 42 mm; the cut edges are rounded and electrode to oxidize and reduce the supporting electrolyte to 1
polished smooth. Other cells conforming to these dimensions to 2 µA in about 3 min with the current following an
are satisfactory. exponential curve.) If such electrode response is not obtained,
6.3.2 CounterElectrodeandSaltBridgeTube—Thecounter the following electrode reconditioning treatments, in increas-
electrode is a coiled length of 0.51-mm (0.020-in.) diameter ing order of severity, have been found to be successful in
platinumwire.Thesaltbridgetubeisunfiredhigh-silicaglass restoring response.
filled with the supporting electrolyte solution. 6.3.4.1 Thegoldelectrodemaybe:(1)brieflydippedincold
6.3.3 Reference Electrode and Salt Bridge Tube—The ref- concentrated HCl and thoroughly rinsed with 8 M HNO;(2)
erence electrode is a miniature saturated-calomel electrode briefly dipped in hot HCl and thoroughly rinsed with HNO ;
(3) briefly dipped in aqua regia and thoroughly rinsed with
HNO;or(4) soaked 10 min in the sulfuric acid-hydrofluoric
EitheratesttubewithunfiredVycorbottomsofType7930glassobtainedfrom acid mixture (7.16), the residual acid removed by fuming and
Corning Glass Works, or a 0.5 cm long, 0.5-cm diameter rod of unfired Vycor Type
thehotelectrodequenchedin8 MHNO .Aftereachtreatment,
7930 sealed into one end of a glass tube with heat-shrinkable TFE-fluorocarbon
the electrode is stored in 8 M HNO overnight. Following
tubing, has been found satisfactory for this application.
overnight storage, conditioning, that is, alternating reduction
AFisher Calomel Reference Electrode Catalog No. 13-639-79 has been found
satisfactory.
FIG. 1 Exploded View of Cell Assembly: (a) Counter Electrode,
(b) Cell Head, (c) Counter Electrode Frit Tube, (d) Reference
Electrode Frit Tube, (e) NBL-Designed S-Shaped Stirrer, (f)
Working Electrode, (g) Sample Cell, (h) Stirrer Motor, (i) Motor
Pedestal and Bearing, and (j) Stirrer Shaft FIG. 2 Working Electrode (Top View)
C 1108
and oxidation of the supporting electrolyte with and without obtained using overhead heating with quartz heat lamps
plutonium, may be required to achieve desired electrode controlled by a variable power supply. However, with proper
performance. care, other conventional means of heating may be used.
6.3.4.2 The platinum electrode may be subjected to any of 6.5 Hot Plate—Recommended for heating during the plu-
the above treatments, or it may be: (1) heated to red heat in a tonium oxidation state adjustment with hydrogen peroxide.
gasflameandquenchedin8MHNO or(2)heatedinafurnace 6.6 Quartz Clock Timer, accurate to 0.001 s.
to 900°C and quenched in 8 M HNO . Do not use these latter 6.7 100-V Precision Resistor, accurate to better than
treatments on gold electrodes as melting may occur. 0.01%.
6.3.5 Stirrer—Several types of stirrers have performed sat-
7. Reagents and Materials
isfactorily. A paddle-type stirrer capable of being driven at
1800 r/min by a synchronous motor, or a magnetically driven 7.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
stirring bar, is adequate. Magnetic stirring slightly simplifies
thearrangementofthecellcap.Foroptimumstirringefficiency all reagents conform to the specifications of the Committee on
Analytical Reagents of theAmerican Chemical Society where
with freedom from losses due to splashing, an S-shap
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.