ASTM C968-99
(Test Method)Standard Test Methods for Analysis of Sintered Gadolinium Oxide-Uranium Dioxide Pellets
Standard Test Methods for Analysis of Sintered Gadolinium Oxide-Uranium Dioxide Pellets
SCOPE
1.1 These test methods cover procedures for the analysis of sintered gadolinium oxide-uranium dioxide pellets to determine compliance with specifications.
1.2 The analytical procedures appear in the following order: Sections Carbon (Total) by Direct Combustion-Thermal Conductivity Method 6 to 15 Chlorine and Fluorine by Pyrohydrolysis Ion-Selective Electrode Method 16 to 22 Gadolinis Content by Energy-Dispersive X-Ray Spectrometry 23 to 32 Hydrogen by Inert Gas Fusion 33 to 40 Isotopic Uranium Composition by Multiple-Filament Surface- Ionization Mass Spectrometric Method 41 to 49 Nitrogen by Distillation-Nessler Reagent (Photometric) Method 50 to 60 Oxygen-to-Metal Ratio of Sinterod Gadolinium Oxide-Uranium Dioxide Pellets 61 to 70 Spectrochemical Determination of Trace Impurity Elements 71 to 77 Total Gas by Hot Vacuum Extraction 78 to 85 Ceramographic Determination of Free Gd2O3 and Free UO2 to Estimate the Homogeneity of (U,Gd)O2 Pellets 86 to 93
1.3 The values stated in SI units are to be regarded as the standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:C968–99
Standard Test Methods for
Analysis of Sintered Gadolinium Oxide-Uranium Dioxide
Pellets
This standard is issued under the fixed designation C968; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope C889 TestMethodsforChemicalandMassSpectrographic
Analysis of Nuclear-Grade Gadolinium Oxide (Gd O )
2 3
1.1 These test methods cover procedures for the analysis of
Powder
sintered gadolinium oxide-uranium dioxide pellets to deter-
C 922 Specification for Sintered Gadolinium Oxide-
mine compliance with specifications.
Uranium Dioxide Pellets
1.2 Theanalyticalproceduresappearinthefollowingorder:
C1347 PracticeforPreparationandDissolutionofUranium
Sections
Materials for Analysis
Carbon (Total) by Direct Combustion—Thermal Conductivity
Method
C1408 Test Method for Carbon (Total) in Uranium Oxide
C 1408 Test Method for Carbon (Total) in Uranium Oxide Pow-
Powders and Pellets By Direct Combustion-Infrared De-
ders and Pellets By Direct Combustion-Infrared Detection
tection Method
Method
Chlorine and Fluorine by Pyrohydrolysis Ion-Selective Elec- 16 to 22
C1413 Test Method for Isotopic Analysis Of Hydrolysed
trode Method
Uranium Hexafluoride And Uranyl Nitrate Solutions By
Gadolinia Content by Energy-Dispersive X-Ray Spectrometry 23 to 32
Hydrogen by Inert Gas Fusion 33 to 40 Thermal Ionization Mass Spectrometry
Isotopic Uranium Composition by Multiple-Filament Surface-
D1193 Specification for Reagent Water
Ionization Mass Spectrometric Method
3 E115 Practice for Photographic Processing in Optical
C 1413 Test Method for Isotopic Analysis Of Hydrolysed Uranium
Hexafluoride And Uranyl Nitrate Solutions By Thermal Ioniza- Emission Spectrographic Analysis
tion Mass Spectrometry
E116 Practice for Photographic Photometry in Spectro-
Nitrogen by Distillation—Nessler Reagent (Photometric) Method 50 to 60
chemical Analysis
Oxygen-to-Metal Ratio of Sintered Gadolinium Oxide-Uranium 61 to 70
Dioxide Pellets
E130 Practice for Designation of Shapes and Sizes of
Spectrochemical Determination of Trace Impurity Elements 71 to 77
Graphite Electrodes
Total Gas by Hot Vacuum Extraction
E146 Methods for Chemical Analysis of Zirconium and
Ceramographic Determination of Free Gd O and Free UO to 86 to 93
2 3 2
Estimate the Homogeneity of (U,Gd)O Pellets
Zirconium Alloys
1.3 The values stated in SI units are to be regarded as the
3. Significance and Use
standard.
3.1 The test methods in this method are designed to show
1.4 This standard does not purport to address all of the
whether a given material is in accordance with Specification
safety concerns, if any, associated with its use. It is the
C922.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
4. Reagents
bility of regulatory limitations prior to use.
4.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
2. Referenced Documents
all reagents shall conform to the specifications of the commit-
2.1 ASTM Standards:
tee on Analytical Reagent of the American Chemical Society,
C696 TestMethodsforChemical,MassSpectrometric,and
where such specifications are available. Other grades may be
Spectrochemical Analysis of Nuclear-Grade Uranium Di-
used, provided it is first ascertained that the reagent is of
oxide Powders and Pellets
Annual Book of ASTM Standards, Vol 11.01.
1 5
These test methods are under the jurisdiction of ASTM Committee C-26 on Annual Book of ASTM Standards, Vol 03.05.
NuclearFuelCycleandarethecompleteresponsibilityofSubcommitteeC26.05on Reagent Chemicals, American Chemical Society Specifications, American
Test Methods. Chemical Society, Washington, DC. For suggestions on the testing of reagents not
Current edition approved Jan. 10, 1999. Published March 1999. Originally listed by the American Chemical Society, see Analar Standards for Laboratory
published as C968–81. Last previous edition C968–94. Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
Discontinued 1999. See C968–94 and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
Annual Book of ASTM Standards, Vol 12.01. MD.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
C968
sufficiently high purity to permit its use without lessening the 8.1.3 Combustion Tube Furnace, having a bore of about 32
accuracy of the determination. mm (1 ⁄4in.), a length of about 305 mm (12 in.), and the
4.2 Purity of Water—Unlessotherwiseindicated,references capability of maintaining a temperature of 1000°C.
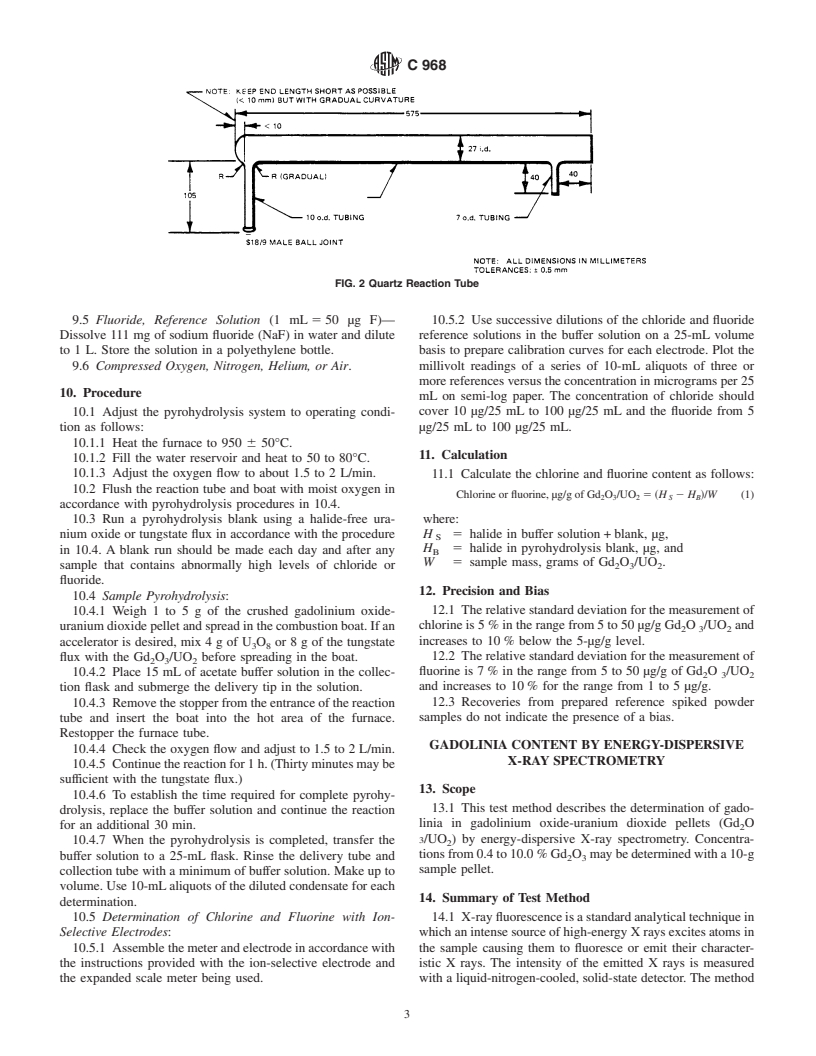
towatershallbeunderstoodtomeanreagentwaterconforming 8.1.4 Quartz Reaction Tube (Fig. 2)—The exit end should
to Type IV of Specification D1193. not extend over 51 mm (2 in.) beyond the furnace with a
ground joint connecting to the delivery tube.The delivery tube
5. Safety Precautions
extendsintoapolyethyleneabsorptionvesselwithatipcapable
5.1 Properprecautionsshouldbetakentopreventinhalation of giving a stream of fine bubbles.
or ingestion of gadolinium oxide or uranium dioxide dust 8.1.5 Combustion Boat—A ceramic, platinum, or quartz
during grinding or handling operations. boat with a 10-mL capacity, 89 to 102 mm (3 ⁄2 to 4 in.) long,
1 3
12.7 mm ( ⁄2 in.) wide, and 9.53 mm ( ⁄8 in.) high.
CARBON (TOTAL) BY DIRECT COMBUSTION—
8.1.6 Absorption Vessel—A 50-mL polyethylene graduate
THERMAL CONDUCTIVITY METHOD
or tube is satisfactory.
This Test Method was discontinued in January 1999 and
8.2 Ion-Selective Electrodes—A chloride-ion-selective ac-
8 9
replaced by Test Method C1408
tivityelectrode andafluoride-ion-selectiveactivityelectrode.
8.3 pH Meter and Double-Junction Reference Electrode,
CHLORINE AND FLUORINE BY PYROHYDROLYSIS
such as a mercuric sulfate, sleeve-junction type. The meter
ION-SELECTIVE ELECTRODE METHOD
should have an expandable scale with a sensitivity of 1 mV.
8.4 Magnetic Stirrer.
6. Scope
8.5 Beakers, 50-mL, polyethylene.
6.1 Thistestmethoddescribesthedeterminationofchlorine
and fluorine in gadolinium oxide-uranium dioxide pellets (Gd
9. Reagents and Materials
2O /UO ). With a 1 to 10-g sample, concentrations from 5 to
3 2
9.1 Accelerator—U O (halogen-free) can be used, but a
3 8
200 µg of chlorine and 1 to 200 µg of fluorine are determined
flux of sodium tungstate (Na WO ) with tungsten trioxide
2 4
without interference.
(WO ) may be advantageous. (See Test Method C696.)
Special preparation of the mixture is necessary, that is, dehy-
7. Summary of Test Method
drate 165 g of Na WO in a large platinum dish. Transfer the
2 4
7.1 The halogens are separated from the gadolinium oxide-
dried material to a mortar. Add 116 g of WO and grind the
uraniumdioxidepelletsbypyrohydrolysisinaquartztubewith
mixture to ensure good mixing. Transfer the mixture into a
a stream of wet oxygen sparge gas at a temperature of 900 to
platinum dish and heat with a burner for 2 h. Cool the melt,
1000°C (1, 2, 3, 4). Chlorine and fluorine are volatilized
transfer the flux to a mortar, and grind it to a coarse powder.
simultaneously as acids, absorbed in a buffer solution, and
Store the flux in an airtight bottle. Mix about8gofflux with
measured with ion-selective electrodes (4, 5, 6). Chloride can
each sample to be pyrohydrolyzed.
also be determined by amperometric titration.
9.2 Buffer Solution (0.001 N)—Dissolve 0.1 g of potassium
acetate (KC H O ) in water, add 0.050 mLof acetic acid (CH
2 3 2
8. Apparatus
3CO H, sp gr 1.05), and dilute to 1 L.
8.1 Pyrohydrolysis Equipment—A suitable assembly of ap-
9.3 Chloride, Reference Solution (1 mL 5100 µg Cl)—
paratus is shown in Fig. 1.
Dissolve 165 mg of dry sodium chloride (NaCl) in water and
8.1.1 Gas Flow Regulator and Flowmeter.
dilute to 1 L.
8.1.2 Hot Plate, used to warm the water saturating the
9.4 Distilled Water—UseASTMType IVwater as specified
sparge gas 50 to 80°C.
in Specification D1193.
7 The Orion Model No. 96-17 has been found satisfactory.
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
The Orion Method No. 9409 has been found satisfactory.
these methods.
FIG. 1 Pyrohydrolysis of Gadolinium Oxide
C968
FIG. 2 Quartz Reaction Tube
9.5 Fluoride, Reference Solution (1 mL 550 µg F)— 10.5.2 Use successive dilutions of the chloride and fluoride
Dissolve 111 mg of sodium fluoride (NaF) in water and dilute reference solutions in the buffer solution on a 25-mL volume
to 1 L. Store the solution in a polyethylene bottle. basis to prepare calibration curves for each electrode. Plot the
9.6 Compressed Oxygen, Nitrogen, Helium, or Air. millivolt readings of a series of 10-mL aliquots of three or
morereferencesversustheconcentrationinmicrogramsper25
10. Procedure
mL on semi-log paper. The concentration of chloride should
cover 10 µg/25 mL to 100 µg/25 mL and the fluoride from 5
10.1 Adjust the pyrohydrolysis system to operating condi-
tion as follows: µg/25 mL to 100 µg/25 mL.
10.1.1 Heat the furnace to 950 6 50°C.
11. Calculation
10.1.2 Fill the water reservoir and heat to 50 to 80°C.
10.1.3 Adjust the oxygen flow to about 1.5 to 2 L/min.
11.1 Calculate the chlorine and fluorine content as follows:
10.2 Flush the reaction tube and boat with moist oxygen in
Chlorineorfluorine,µg/gofGd O /UO 5 ~H 2 H !/W (1)
2 3 2 S B
accordance with pyrohydrolysis procedures in 10.4.
10.3 Run a pyrohydrolysis blank using a halide-free ura- where:
nium oxide or tungstate flux in accordance with the procedure H 5 halide in buffer solution+blank, µg,
S
H 5 halide in pyrohydrolysis blank, µg, and
in 10.4. A blank run should be made each day and after any
B
W 5 sample mass, grams of Gd O /UO .
sample that contains abnormally high levels of chloride or 2 3 2
fluoride.
12. Precision and Bias
10.4 Sample Pyrohydrolysis:
12.1 Therelativestandarddeviationforthemeasurementof
10.4.1 Weigh 1 to5gofthe crushed gadolinium oxide-
chlorineis5%intherangefrom5to50µg/gGd O /UO and
uraniumdioxidepelletandspreadinthecombustionboat.Ifan
2 3 2
increases to 10% below the 5-µg/g level.
accelerator is desired, mix4gofU O or 8 g of the tungstate
3 8
12.2 Therelativestandarddeviationforthemeasurementof
flux with the Gd O /UO before spreading in the boat.
2 3 2
fluorine is 7% in the range from 5 to 50 µg/g of Gd O /UO
10.4.2 Place 15 mL of acetate buffer solution in the collec-
2 3 2
and increases to 10% for the range from 1 to 5 µg/g.
tion flask and submerge the delivery tip in the solution.
12.3 Recoveries from prepared reference spiked powder
10.4.3 Removethestopperfromtheentranceofthereaction
samples do not indicate the presence of a bias.
tube and insert the boat into the hot area of the furnace.
Restopper the furnace tube.
GADOLINIA CONTENT BY ENERGY-DISPERSIVE
10.4.4 Check the oxygen flow and adjust to 1.5 to 2 L/min.
X-RAY SPECTROMETRY
10.4.5 Continuethereactionfor1h.(Thirtyminutesmaybe
sufficient with the tungstate flux.)
13. Scope
10.4.6 To establish the time required for complete pyrohy-
13.1 This test method describes the determination of gado-
drolysis, replace the buffer solution and continue the reaction
linia in gadolinium oxide-uranium dioxide pellets (Gd O
for an additional 30 min.
3/UO ) by energy-dispersive X-ray spectrometry. Concentra-
10.4.7 When the pyrohydrolysis is completed, transfer the
tionsfrom0.4to10.0%Gd O maybedeterminedwitha10-g
buffer solution to a 25-mL flask. Rinse the delivery tube and
2 3
sample pellet.
collection tube with a minimum of buffer solution. Make up to
volume.Use10-mLaliquotsofthedilutedcondensateforeach
14. Summary of Test Method
determination.
10.5 Determination of Chlorine and Fluorine with Ion- 14.1 X-rayfluorescenceisastandardanalyticaltechniquein
Selective Electrodes: whichanintensesourceofhigh-energyXraysexcitesatomsin
10.5.1 Assemblethemeterandelectrodeinaccordancewith the sample causing them to fluoresce or emit their character-
the instructions provided with the ion-selective electrode and istic X rays. The intensity of the emitted X rays is measured
the expanded scale meter being used. with a liquid-nitrogen-cooled, solid-state detector. The method
C968
is calibrated by comparing the measured intensity with that 17. Calibration Reference Materials
produced by reference materials of known gadolinia concen-
17.1 Pellet reference materials covering the weight percent
tration at an averaged Ka peak of 42.76 keV (7, 8).
range of interest must be carefully prepared. X-ray fluores-
14.2 This determination is carried out on an energy-
cence excites only the surface atoms; hence differences in
dispersive X-ray spectrometer, where the radiation from an
gadolinium content within each pellet must be no greater than
americium-241 or other source is used to activate the second-
1%. Gadolinia is hygroscopic and must be heated to assure
ary radiation due to gadolinium and uranium.These secondary
#1% water retention by weight when reference materials are
radiations can be detected with Si(Li), Ge(Li), or an intrinsic
initially prepared by blending weighed amounts of Gd O and
2 3
germanium solid state detector maintained at liquid nitrogen
UO .
temperatures. By means of a single-channel analyzer the
17.2 The gadolinia content of the sintered pellet reference
radiationisselectedsoastorecordonlythoseradiationsdueto
materials should be independently verified by another analyti-
the Ka radiation of gadolinium.
cal method such as by oxalate precipitation (9).
14.3 This analysis may also be performed by wavelength
18. Reagents and Materials
dispersive X-ray analysis. The user must demonstrate the
equivalency to the energy dispersive method.
18.1 Liquid Nitrogen.
18.2 Plastic Rings, 25.4 mm (1-in.) diameter.
15. Interferences
18.3 Cap Plugs, 25.4 mm (1-in.) diameter.
15.1 Rareearthsinterferewhenconcentrationsareinexcess
18.4 Isopropyl Alcohol.
of 1% of Gd O /UO .
18.5 Epoxy Resin and Hardener.
2 3 2
18.6 Grinding Disks, 320, 400, 600-grit.
16. Apparatus
18.7 Gadolinium Oxide-Uranium Dioxide Reference
16.1 Solid-State X-Ray Detector,$30mm inarea,$5mm
Pellets—Accurately prepare a series of working reference
in thickness, with a resolution of 0.2 keV at 45 keV.
(Gd O /UO ) sintered pellets covering the range of gadolinia
2 3 2
16.2 Energy-Dispersive X-Ray Spectrometer System—See
concentrations anticipated in the pellets to be tested, using the
Fig. 3.
purest gadolinia and urania available.
16.3 Cryogenic Subsyst
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.