ASTM D2712-91(2003)e1
(Test Method)Standard Test Method for Hydrocarbon Traces in Propylene Concentrates By Gas Chromatography
Standard Test Method for Hydrocarbon Traces in Propylene Concentrates By Gas Chromatography
SIGNIFICANCE AND USE
The trace hydrocarbon compounds listed in Table 1 may have an effect in the commercial use of propylene concentrates, and information on their concentration is frequently necessary.
TABLE 1 Molecular Weight and Specific Gravity CompoundMolecular WeightSpecific Gravity, 60/60 Propylene 42.080.5220 Propane44.090.5077
SCOPE
1.1 This test method covers the determination of 5 to 500 ppm each of ethylene, total butylenes, acetylene, methyl acetylene, propadiene, and butadiene in propylene concentrates.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parenthese are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information.
´1
Designation: D2712 – 91 (Reapproved 2003)
Standard Test Method for
Hydrocarbon Traces in Propylene Concentrates by Gas
Chromatography
This standard is issued under the fixed designation D2712; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Warning notes were editorially moved into the standard text in September 2003.
1. Scope 4. Significance and Use
1.1 This test method covers the determination of 5 to 500 4.1 The trace hydrocarbon compounds listed inTable 1 may
ppm each of ethylene, total butylenes, acetylene, methyl haveaneffectinthecommercialuseofpropyleneconcentrates,
acetylene, propadiene, and butadiene in propylene concen- and information on their concentration is frequently necessary.
trates.
5. Apparatus
1.2 The values stated in SI units are to be regarded as
5.1 Columns—Any column may be used provided it will
standard. The values given in parentheses are for information
only. resolve the trace compound peaks present in concentrations of
20 ppm or more so that the resolution ratio, A/B, will not be
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the less than 0.4, where A is the depth of the valley on either side
of peak B and B is the height above the baseline of the smaller
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- of any two adjacent peaks (see Fig. 1). For compounds present
in concentrations of less than 20 ppm the ratioA/B may be less
bility of regulatory limitations prior to use.
than 0.4. In the case where the small-component peak is
2. Referenced Documents
adjacent to a large one, it may be necessary to construct the
2.1 ASTM Standards: baseline of the small peak tangent to the curve as shown in Fig.
E260 Practice for Packed Column Gas Chromatography 2. Butylenes need not be resolved from each other. Columns
F307 Practice for Sampling Pressurized Gas for GasAnaly- found to be acceptable together with operating conditions used
sis are shown in Table 2. Table 3 shows typical retention times.
5.1.1 Columns may be constructed of 3.2-mm ( ⁄8-in.),
3. Summary of Test Method
6.4-mm ( ⁄4-in.), or capillary tubing and usually need to be a
3.1 A relatively large volume of sample is charged to a gas minimum of 6 m (20 ft) in length. They usually have 20 to 40
partition chromatography apparatus which has a column that
g of liquid substrate to 100 g of solid support. If packed
will separate the trace hydrocarbon constituents from the major columnsareused,theliquidmaybeplacedonthesolidsupport
components. Any column or combination of columns may be
by any suitable method, provided the column has the desired
used provided they have the necessary resolution and the resolution and sensitivity.
detectingsystemhassufficientsensitivity.Severalcolumnsthat
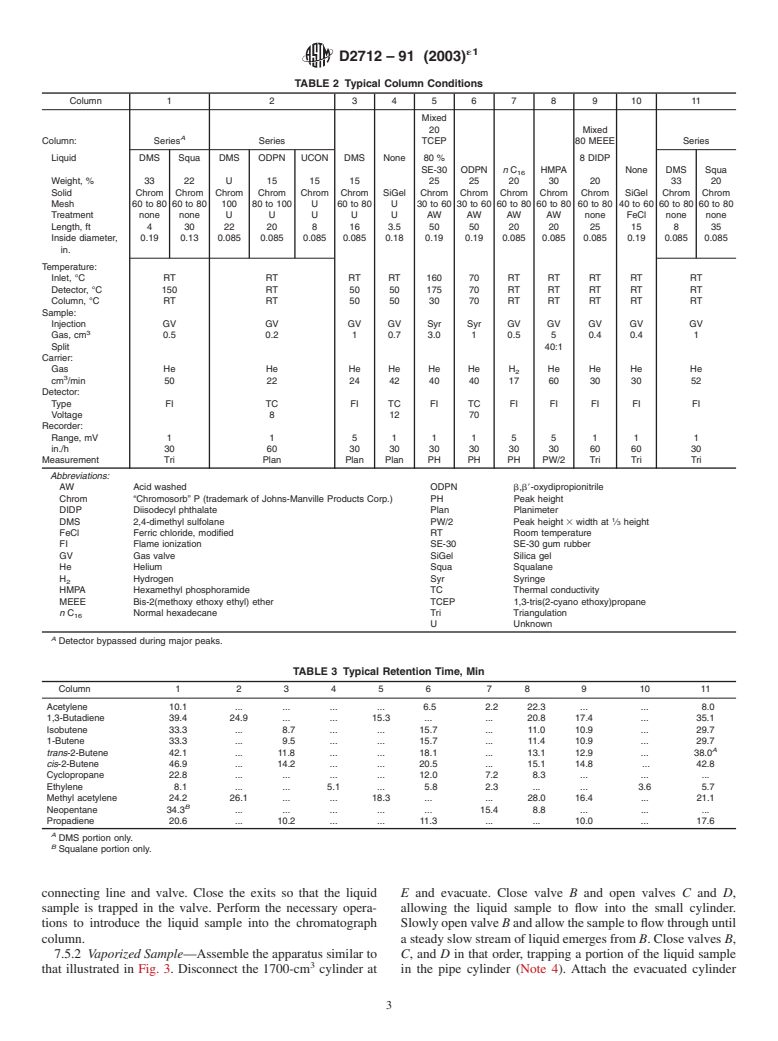
NOTE 1—Separation of all the desired compounds on a single column
have been found satisfactory are given in 5.1.
has been found by cooperators to be very difficult. Most laboratories have
3.2 Calculation is performed by calculating the concentra-
found it necessary to use two or more columns. Typical instructions for
tion of the trace compound from its area relative to the area of
preparing such columns may be found in Practice E260.
a standard compound of known concentration.
5.2 Gas Chromatograph—Any gas chromatography appa-
ratus may be used provided the system has sufficient sensitivity
to detect the trace compounds of interest. For calculation
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
techniques utilizing a recorder, the signal for 20 ppm concen-
D02.D0 on Hydrocarbons for Chemical and Special Uses.
tration shall be at least 5 chart divisions above the noise level
Current edition approved Sept. 10, 2003. Published September 2003. Originally
ona0to100 scale chart. The noise level must be restricted to
approved in 1968. Last previous edition approved in 1996 as D2712–91(1996).
DOI: 10.1520/D2712-91R03E01.
Annual Book of ASTM Standards, Vol 14.02.
Annual Book of ASTM Standards, Vol 15.03.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
D2712 – 91 (2003)
TABLE 1 Molecular Weight and Specific Gravity
6.2 Propane or Propylene, for synthetic base stock contain-
Compound Molecular Weight Specific Gravity, 60/60 ing less than 2 ppm by weight of acetylene or 1,3-butadiene.
Propylene 42.08 0.5220
(Warning—Liquefied petroleum gas under pressure and flam-
Propane 44.09 0.5077
mable.)
6.3 Calibration Compounds—Acetylene and 1,3-butadiene
99 % minimum purity. (Warning—Liquefied petroleum gas
under pressure and flammable.)
6.4 Carrier Gases—Helium or Nitrogen.(Warning—
Compressed gas under pressure.)
6.5 Hydrogen.(Warning—Compressed gas under pressure
and flammable.)
6.6 Liquid Phase for Column—See Table 2.(Warning—
Hexamethylphosphoramide is a potential carcinogen.)
6.7 Solid Support—C firebrick or diatomaceous earth,
usually 40 to 60 or 60 to 80 mesh.
6.8 Stainless Steel Sample Cylinder, 300 to 500-cm capac-
ity, capable of withstanding a minimum of 1723 kPa gage (250
psig).
FIG. 1 Illustration of A/B Ratio
6.9 SiliconeRubberSeptum,withsuitablefittingsforattach-
ment to sample cylinder.
6.10 Gas Syringe, 10-cm .
6.11 Vacuum Pump, capable of evacuating sample cylinder
to less than 2 mm Hg absolute pressure.
6.12 AluminumorStainlessSteelTubing, 0.61 m (2 ft), 3.2
1 1
mm ( ⁄8 in.), or 1.6 mm ( ⁄16 in.), outside diameter with fittings
on one end to connect to butadiene cylinder and the other end
modified so as to have an opening with an inside diameter of
about 0.5 mm larger than the outside diameter of the gas
syringe needle.
7. Sampling
7.1 This section is to be followed on all samples including
FIG. 2 Illustration of A/B Ratio for Small-Component Peak
unknown samples and the synthetic standards.
7.2 Samples should be supplied to the laboratory in high-
pressure sample cylinders, obtained using the procedures
a maximum of 2 chart divisions.When electronic integration is described in Practice F307 or similar methods.
employed, the signal for 20-ppm concentration must be at least
7.3 Place the cylinder in a horizontal position in a safe
twice the noise level. location such as a hood. Check to see that the container is at
least one-half full by slightly opening the valve. If liquid is
NOTE 2—A flame ionization detector is preferred. When using with
emitted (a white cloud of vapors) the container is at least
relatively volatile liquid phases, such as HMPA, an additional 0.31-m
one-half full. Do not analyze any samples or use any synthetic
(1-ft) section of column containing uncoated solid support will aid in
standard if the liquid in the container is less than this amount.
reducing noise.
7.4 Place the cylinder in a vertical position and repressurize
5.3 Sample Introduction—Means shall be provided for in-
to 1723 kPa gage (250 psig) with the chromatographic carrier
troducing a measured quantity of sample into the apparatus.
gas through the valve at the top of the cylinder, ensuring that
Pressure sampling devices may be used to inject a small no air enters during the operation.
7.5 Use either of the following two procedures for obtaining
amount of the liquid directly into the carrier gas. Introduction
a sample from the container:
may be by means of a gas valve to charge the vaporized liquid.
7.5.1 Using a Liquid Valve—Connect the cylinder to the
liquid valve on the chromatograph using a minimum length of
6. Reagents and Materials
connecting tubing, so that sample is withdrawn from the
6.1 Hydrocarbons, for peak identification, including propy-
bottom of the cylinder and a liquid sample is obtained. The
lene, ethylene, ethane, acetylene, methyl acetylene, propadi-
liquid valve on the chromatograph must be designed in such a
ene, propane, 1,3-butadiene, isobutylene, 1-butene, cis and
mannerthatfullsamplepressurecanbemaintainedthroughthe
trans 2-butene, iso- and normal butane, and cyclopropane.
valve without leaking and that means are provided for trapping
(Warning—Liquefied petroleum gas under pressure and flam-
a liquid sample in the chromatograph valve under static
mable.) Mixtures of these hydrocarbons may be used for
conditions of flow. With the exit of the chromatograph valve
calibration provided there is no uncertainty as to the identity of
closed open the valve on the cylinder. Slowly open the exit
the desired compound. from the chromatograph valve so that liquid flows through the
´1
D2712 – 91 (2003)
TABLE 2 Typical Column Conditions
Column 1 2 345678 9 10 11
Mixed
20 Mixed
A
Column: Series Series TCEP 80 MEEE Series
Liquid DMS Squa DMS ODPN UCON DMS None 80 % 8 DIDP
SE-30 ODPN n C HMPA None DMS Squa
Weight,% 33 22 U 15 1515 25252030 20 3320
Solid Chrom Chrom Chrom Chrom Chrom Chrom SiGel Chrom Chrom Chrom Chrom Chrom SiGel Chrom Chrom
Mesh 60 to 80 60 to 80 100 80 to 100 U 60 to 80 U 30 to 60 30 to 60 60 to 80 60 to 80 60 to 80 40 to 60 60 to 80 60 to 80
Treatment none none U U U U U AW AW AW AW none FeCl none none
Length, ft 4 30 22 20 8 16 3.5 50 50 20 20 25 15 8 35
Inside diameter, 0.19 0.13 0.085 0.085 0.085 0.085 0.18 0.19 0.19 0.085 0.085 0.085 0.19 0.085 0.085
in.
Temperature:
Inlet, °C RT RT RT RT 160 70 RT RT RT RT RT
Detector, °C 150 RT 50 50 175 70 RT RT RT RT RT
Column, °C RT RT 50 50 30 70 RT RT RT RT RT
Sample:
Injection GV GV GV GV Syr Syr GV GV GV GV GV
Gas, cm 0.5 0.2 1 0.7 3.0 1 0.5 5 0.4 0.4 1
Split 40:1
Carrier:
Gas He He He He He He H He He He He
cm /min 50 22 24 42 40 40 17 60 30 30 52
Detector:
Type FI TC FI TC FI TC FI FI FI FI FI
Voltage 8 12 70
Recorder:
Range, mV 1 1 511155 1 1 1
in./h 30 60 30 30 30 30 30 30 60 60 30
Measurement Tri Plan Plan Plan PH PH PH PW/2 Tri Tri Tri
Abbreviations:
AW Acid washed ODPN b,b8-oxydipropionitrile
Chrom “Chromosorb” P (trademark of Johns-Manville Products Corp.) PH Peak height
DIDP Diisodecyl phthalate Plan Planimeter
DMS 2,4-dimethyl sulfolane PW/2 Peak height 3 width at ⁄3 height
FeCl Ferric chloride, modified RT Room temperature
FI Flame ionization SE-30 SE-30 gum rubber
GV Gas val
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.