ASTM E2196-07
(Test Method)Standard Test Method for Quantification of a Pseudomonas aeruginosa Biofilm Grown with Shear and Continuous Flow Using a Rotating Disk Reactor
Standard Test Method for Quantification of a <i>Pseudomonas aeruginosa</i> Biofilm Grown with Shear and Continuous Flow Using a Rotating Disk Reactor
SIGNIFICANCE AND USE
Bacteria that exist in a biofilm are phenotypically different from suspended cells of the same genotype. The study of biofilm in the laboratory requires protocols that account for this difference. Laboratory biofilms are engineered in growth reactors designed to produce a specific biofilm type. Altering system parameters will correspondingly result in a change in the biofilm. The purpose of this method is to direct a user in the laboratory study of biofilms by clearly defining each system parameter. This method will enable a person to grow, sample, and analyze a laboratory biofilm.
SCOPE
1.1 This test method is used for growing a repeatable Pseudomonas aeruginosa biofilm in a continuously stirred flow reactor. In addition, the test method describes how to sample and analyze biofilm for viable cells.
1.2 In this test method, biofilm population density is recorded as log colony forming units per surface area.
1.3 Basic microbiology training is required to perform this test method. This standard does not claim to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
Designation: E2196 – 07
Standard Test Method for
Quantification of a Pseudomonas aeruginosa Biofilm Grown
with Shear and Continuous Flow Using a Rotating Disk
1
Reactor
This standard is issued under the fixed designation E2196; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope other, embedded in a matrix of extracellular polymeric sub-
2 stances of microbial origin, while exhibiting an altered pheno-
1.1 This test method is used for growing a repeatable
type with respect to growth rate and gene transcription.
Pseudomonasaeruginosabiofilminacontinuouslystirredflow
3.1.1 Discussion—Biofilms may be comprised of bacteria,
reactor. In addition, the test method describes how to sample
fungi, algae, protozoa, viruses, or infinite combinations of
and analyze biofilm for viable cells.
these microorganisms. The qualitative characteristics of a
1.2 In this test method, biofilm population density is re-
biofilm, including, but not limited to, population density,
corded as log colony forming units per surface area.
taxonomic diversity, thickness, chemical gradients, chemical
1.3 Basic microbiology training is required to perform this
composition,consistency,andothermaterialsinthematrixthat
test method. This standard does not claim to address all of the
arenotproducedbythebiofilmmicroorganisms;arecontrolled
safety problems associated with its use. It is the responsibility
by the physiochemical environment in which it exists.
of the user of this standard to establish appropriate safety
3.2 coupon—biofilm sample surface.
practices and determine the applicability of regulatory limita-
tions prior to use.
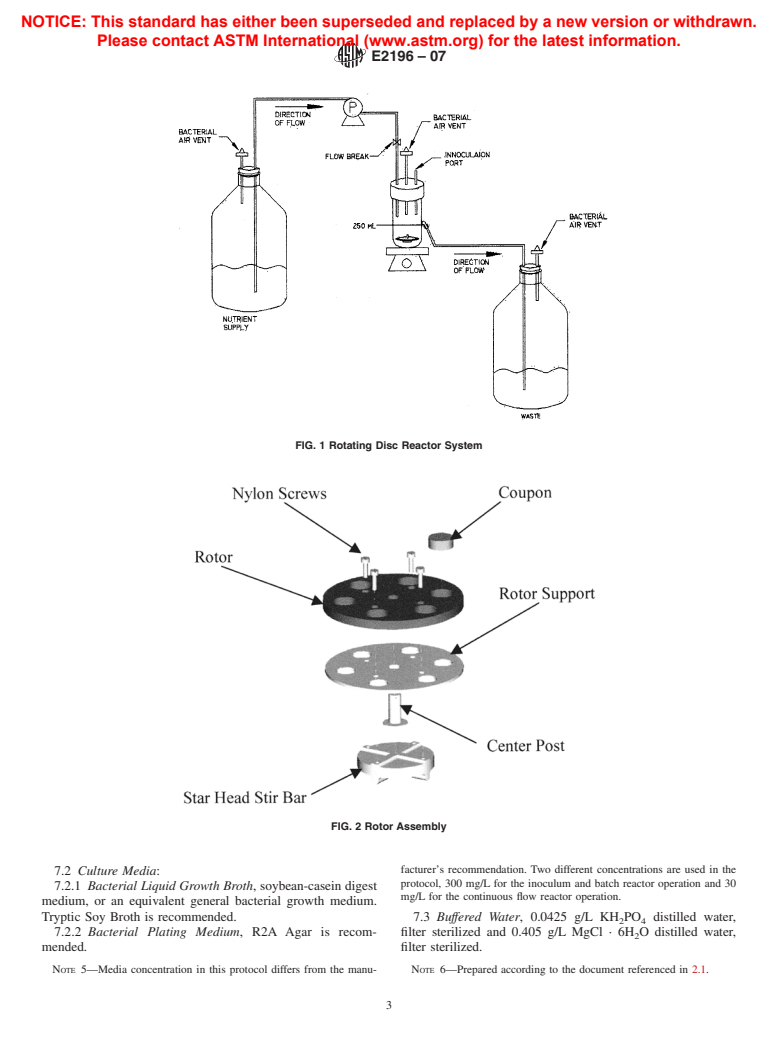
4. Summary of Test Method
2. Referenced Documents 4.1 This test method is used for growing a repeatable
Pseudomonas aeruginosa biofilm in a rotating disk reactor.
2.1 Other Standards:
3
The biofilm is established by operating the reactor in batch
Buffered Dilution Water Preparation —Method 9050 C.1a
4
mode (no flow) for 24 h. A steady state growth (attachment is
Rotating Disk Reactor —Repeatability and Relevance
5
equal to detachment) is reached while the reactor operates for
Rotating Disk Reactor —Efficacy Test Method
an additional 24 h with continuous flow of the nutrients. The
3. Terminology
residence time of the nutrients in the reactor is set to select for
biofilm growth, and is species and reactor parameter specific.
3.1 biofilm, n—microorganisms living in a self-organized,
During the entire 48 h, the biofilm is exposed to continuous
cooperativecommunityattachedtosurfaces,interfaces,oreach
fluid shear from the rotation of the disk.At the end of the 48 h,
biofilm accumulation is quantified by removing coupons from
1
This test method is under the jurisdiction of ASTM Committee E35 on
the disk, scraping the biofilm from the coupon surface,
Pesticides and Alternative Control Agents and is the direct responsibility of
disaggregating the clumps, then diluting and plating for viable
Subcommittee E35.15 on Antimicrobial Agents.
cell enumeration.
Current edition approved April 1, 2007. Published May 2007. Originally
approved in 2002. Last previous edition approved in 2002 as E2196 – 02. DOI:
10.1520/E2196-07.
5. Significance and Use
2
Ellison, S.L.R., M. Rosslein, A. Williams. (Eds.) 2000. Quantifying Uncer-
5.1 Bacteria that exist in a biofilm are phenotypically
tainty in Anyalytical Measurement, 2nd Edition. Eurachem.
3
Eaton, A.D., L.S. Clesceri, Rice, E.W., A.E. Greenberg. (Eds.) Standard different from suspended cells of the same genotype.The study
Methods for the Examination of Water and Waste Water , 21st Edition. American
of biofilm in the laboratory requires protocols that account for
Public HealthAssociation,American Water WorksAssociation, Water Environment
this difference. Laboratory biofilms are engineered in growth
Federation. Washington D.C., 2005.
4
reactors designed to produce a specific biofilm type. Altering
Zelver, N., M. Hamilton, B. Pitts, D. Goeres, D. Walker, P. Sturman, J.
Heersink. 1999. Methods for measuring antimicrobial effects on biofilm bacteria:
system parameters will correspondingly result in a change in
from laboratory to field. In: Doyle, R.J. (Ed.), Methods in Enzymology-Biofilms Vol
thebiofilm.Thepurposeofthismethodistodirectauserinthe
310, Academic Press, San Diego, CA, pp. 608-628.
5 laboratory study of biofilms by clearly defining each system
Zelver, N., M. Hamilton, D. Goeres, J. Heersink. 2001. Development of a
Standardized Antibiofilm Test. In: Doyle, R.J. (Ed.), Methods in Enzymology-
Biofilms Vol 337, Academic Press, San Diego, CA, pp. 363-376.
Copyright © ASTM International, 100 Barr Harbor Dri
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.