ASTM D6784-02(2008)
(Test Method)Standard Test Method for Elemental, Oxidized, Particle-Bound and Total Mercury in Flue Gas Generated from Coal-Fired Stationary Sources (Ontario Hydro Method)
Standard Test Method for Elemental, Oxidized, Particle-Bound and Total Mercury in Flue Gas Generated from Coal-Fired Stationary Sources (Ontario Hydro Method)
SIGNIFICANCE AND USE
The measurement of particle-bound, oxidized, elemental, and total mercury in stationary-source flue gases provides data that can be used for dispersion modeling, deposition evaluation, human health and environmental impact assessments, emission reporting, compliance determinations, etc. Particle-bound, oxidized, and elemental mercury measurements before and after control devices may be necessary for optimizing and evaluating the mercury removal efficiency of emission control technologies.
SCOPE
1.1 This method applies to the determination of elemental, oxidized, particle-bound, and total mercury emissions from coal-fired stationary sources.
1.2 This method is applicable to elemental, oxidized, particle-bound, and total mercury concentrations ranging from approximately 0.5 to 100 μg/Nm3.
1.3 This method describes equipment and procedures for obtaining samples from effluent ducts and stacks, equipment and procedures for laboratory analysis, and procedures for calculating results.
1.4 This method is applicable for sampling elemental, oxidized, and particle-bound mercury in flue gases of coal-fired stationary sources. It may not be suitable at all measurement locations, particularly those with high particulate loadings, as explained in Section 17.
1.5 Method applicability is limited to flue gas stream temperatures within the thermal stability range of the sampling probe and filter components.
1.6 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
1.7 This standard assumes users are familiar with EPA stack-gas sampling procedures as stated in EPA Methods 1–4, Method 5, and Method 17.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6784 − 02(Reapproved 2008)
Standard Test Method for
Elemental, Oxidized, Particle-Bound and Total Mercury in
Flue Gas Generated from Coal-Fired Stationary Sources
(Ontario Hydro Method)
This standard is issued under the fixed designation D6784; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This method applies to the determination of elemental,
D1193Specification for Reagent Water
oxidized, particle-bound, and total mercury emissions from
D1356Terminology Relating to Sampling and Analysis of
coal-fired stationary sources.
Atmospheres
1.2 This method is applicable to elemental, oxidized,
D2986Practice for Evaluation of Air Assay Media by the
particle-bound, and total mercury concentrations ranging from Monodisperse DOP (Dioctyl Phthalate) Smoke Test
approximately 0.5 to 100 µg/Nm . (Withdrawn 2004)
D3154Test Method for Average Velocity in a Duct (Pitot
1.3 This method describes equipment and procedures for
Tube Method)
obtaining samples from effluent ducts and stacks, equipment
D3685/D3685MTestMethodsforSamplingandDetermina-
and procedures for laboratory analysis, and procedures for
tion of Particulate Matter in Stack Gases
calculating results.
E1Specification for ASTM Liquid-in-Glass Thermometers
2.2 Other Standards:
1.4 This method is applicable for sampling elemental,
EPAMethod1SampleandVelocityTraversesforStationary
oxidized,andparticle-boundmercuryinfluegasesofcoal-fired
Sources
stationary sources. It may not be suitable at all measurement
EPA Method 2Determination of Stack Gas Velocity and
locations, particularly those with high particulate loadings, as
Volumetric Flow Rate (Type S Pitot Tube)
explained in Section 16.
EPA Method 3Gas Analysis for the Determination of Dry
1.5 Method applicability is limited to flue gas stream Molecular Weight
temperatureswithinthethermalstabilityrangeofthesampling EPA Method 4Determination of Moisture Content in Stack
Gases
probe and filter components.
EPAMethod 5Determination of Particulate Emissions from
1.6 The values stated in SI units are to be regarded as the
Stationary Sources
standard. The values in parentheses are for information only.
EPAMethod 12Determination of Inorganic Lead Emissions
from Stationary Sources
1.7 This standard assumes users are familiar with EPA
EPA Method 17Determination of Particulate Emissions
stack-gas sampling procedures as stated in EPAMethods 1–4,
from Stationary Sources (In-Stack Filtration Method)
Method 5, and Method 17.
EPA Method 29Determination of Metals Emissions from
1.8 This standard does not purport to address all of the
Stationary Sources
safety concerns, if any, associated with its use. It is the
EPA Method 101ADetermination of Particle-Bound and
responsibility of the user of this standard to establish appro-
Gaseous Mercury Emissions from Sewage Sludge Incin-
priate safety and health practices and determine the applica- erators
bility of regulatory limitations prior to use.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This test method is under the jurisdiction of ASTM Committee D22 on Air the ASTM website.
Quality and is the direct responsibility of Subcommittee D22.03 on Ambient The last approved version of this historical standard is referenced on
Atmospheres and Source Emissions. www.astm.org.
Current edition approved April 1, 2008. Published July 2008. Originally Available from the U.S. Environmental Protection Agency’s Emission Mea-
approved in 2002. Last previous edition approved in 2002 as D6784–02. DOI: surement Technical Information Center or Code of Federal Regulations (40 CFR
10.1520/D6784-02R08. Part 60, Appendix A or 40 CFR Part 61, Appendix B).
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6784 − 02 (2008)
EPAMethod 301FieldValidation of Pollutant Measurement P =standard absolute pressure, 101.3 kPa (29.92 in. Hg)
std
Methods from Various Waste Media R=ideal gas constant, 0.008314 kPa-m /K-g-mole (21.85
EPA SW 846 7470AMercury in Liquid Waste—Manual in. Hg-ft /°R-lb-mole)
Cold Vapor Technique T =absolute average dry gas meter temperature, K (°R)
m
EPA Water and Waste 600/4-79-020Methods for Chemical T =absolute stack temperature, K (°R)
s
Analysis of Water and Wastes T =standard absolute temperature, 293 K (528°R)
std
V =total digested volume, mL
D
3. Terminology
V =volume of gas sample as measured by dry gas meter,
m
m (dscf)
3.1 Definitions other than those given below in 3.2 and 3.3
are listed in Terminology D1356. V =volume of gas sample measured by the dry gas
m(std)
meter, corrected to standard conditions, Nm (dscf)
3.2 Definitions of Terms Specific to This Standard:
V =volume of water vapor in the gas sample, corrected
w(std)
3.2.1 elemental mercury—mercury in its zero oxidation
0 to standard conditions, m (scf)
state, Hg .
W =total mass of ash on sample filter, g
ash
3.2.2 elemental mercury catch—mercury collected in the
W =total weight of liquid collected in impingers and silica
lc
acidified hydrogen peroxide (HNO –H O ) and potassium
3 2 2
gel, g (lb)
permanganate (H SO –KMnO ) impinger solutions employed
2 4 4
Y=dry gas meter calibration factor
in this method. This is gaseous Hg .
θ=total sampling time, min
3.2.3 front half of the sampling train—all mercury collected
θ =sampling time interval, from the beginning of a run
on and upstream of the sample filter. until the first component change, min
3.2.4 impinger train—setup including only the impingers
4. Summary of Test Method
and connectors.
4.1 A sample is withdrawn from the flue gas stream isoki-
3.2.5 oxidized mercury—mercury in its mercurous or mer-
2+ 2+
netically through a probe/filter system, maintained at 120°C or
curic oxidation states: Hg and Hg , respectively.
the flue gas temperature, whichever is greater, followed by a
3.2.6 oxidized mercury catch—mercury collected in the
series of impingers in an ice bath. Particle-bound mercury is
aqueouspotassiumchloride(KCl)impingersolutionemployed
collected in the front half of the sampling train. Oxidized
2+
in this method. This is gaseous Hg .
mercury is collected in impingers containing a chilled aqueous
3.2.7 particle-bound mercury catch—mercury associated
potassium chloride solution. Elemental mercury is collected in
with the particulate matter collected in the front half of the
subsequent impingers (one impinger containing a chilled
sampling train.
aqueous acidic solution of hydrogen peroxide and three im-
pingers containing chilled aqueous acidic solutions of potas-
3.2.8 sampletrain—completesetupincludingnozzle,probe,
probe liner, filter, filter holder, impingers, and connectors. sium permanganate). Samples are recovered, digested, and
then analyzed for mercury using cold-vapor atomic absorption
3.2.9 total mercury—all mercury (solid-bound, liquid, or
(CVAAS) or fluorescence spectroscopy (CVAFS).
gaseous)howevergeneratedorentrainedinthefluegasstream
(that is, summation of elemental, oxidized, and particle-bound
5. Significance and Use
mercury).
5.1 The measurement of particle-bound, oxidized,
3.3 Symbols:
elemental, and total mercury in stationary-source flue gases
2 2
A=cross-sectional area of stack, m (ft )
provides data that can be used for dispersion modeling,
B =water vapor in the gas stream, proportion by volume
ws
depositionevaluation,humanhealthandenvironmentalimpact
∆H=average pressure differential across the orifice meter,
assessments, emission reporting, compliance determinations,
kPa (in. H O)
etc. Particle-bound, oxidized, and elemental mercury measure-
Hg =concentration of mercury in sample filter ash, µg/g
ash
tp 3 ments before and after control devices may be necessary for
Hg =concentration of particle-bound mercury, µg/Nm
optimizing and evaluating the mercury removal efficiency of
0 3
Hg =concentration of elemental mercury, µg/Nm
emission control technologies.
2+ 3
Hg =concentration of oxidized mercury, µg/Nm
IR=instrument reading from mercury analyzer, µg/L
6. Interferences
L =leakage rate observed during the post test leak check,
p
6.1 There are no known interferences, but certain biases
m /min (cfm)
may be encountered (see Section 16).
L =maximum acceptable leakage rate
a
M =molecular weight of stack gas, wet basis g/g-mole
s
7. Apparatus
(lb/Lb-mole)
M =molecular weight of water, 18.0 g/g-mole (18.0 lb/Lb- 7.1 Sampling Train—Similar to Test Methods D3685/
w
mole) D3685M, EPAMethod 5/EPAMethod 17 and EPAMethod 29
N=Normal conditions, defined as 0°C and 101.3 kPa, (In trains, as illustrated in Fig. 1.
the U.S. standard conditions 32°F and 1 atmosphere) 7.1.1 Probe Nozzle (Probe Tip)—Glass nozzles are required
P =barometric pressure at the sampling site, kPa (in. Hg) unless alternate nozzles are constructed of materials that are
bar
P =absolute stack gas pressure, kPa (in. Hg) free from contamination and will not interact with the sample.
s
D6784 − 02 (2008)
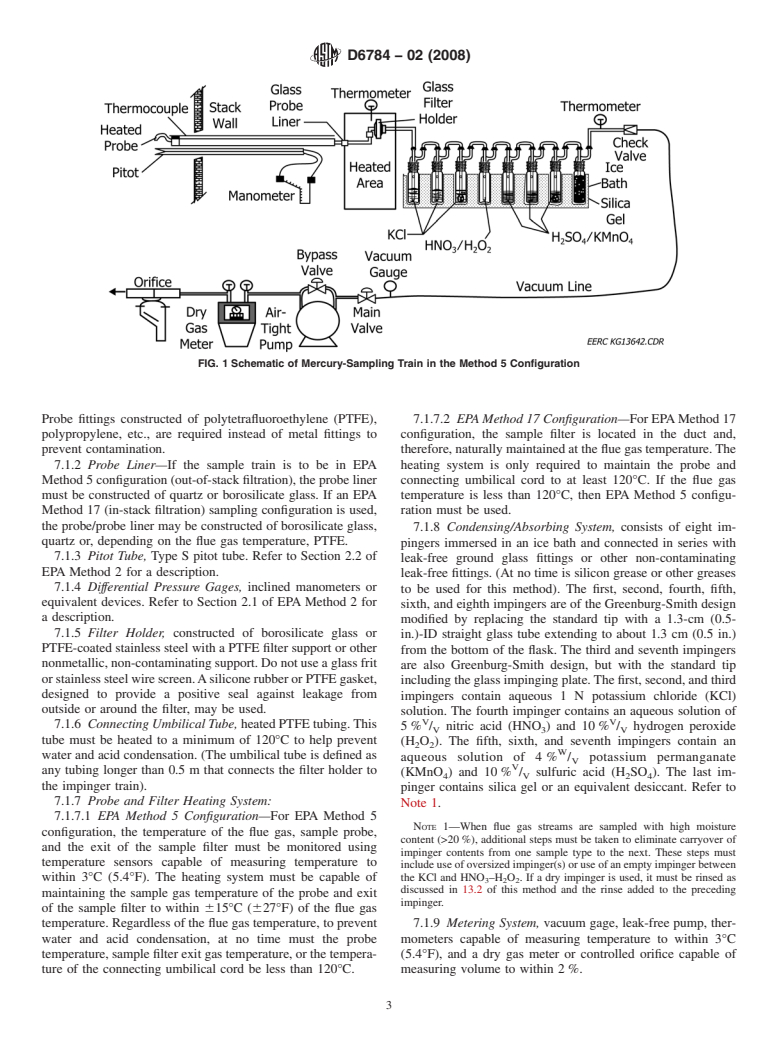
FIG. 1 Schematic of Mercury-Sampling Train in the Method 5 Configuration
Probe fittings constructed of polytetrafluoroethylene (PTFE), 7.1.7.2 EPAMethod 17 Configuration—ForEPAMethod17
polypropylene, etc., are required instead of metal fittings to configuration, the sample filter is located in the duct and,
prevent contamination. therefore,naturallymaintainedatthefluegastemperature.The
7.1.2 Probe Liner—If the sample train is to be in EPA heating system is only required to maintain the probe and
Method5configuration(out-of-stackfiltration),theprobeliner connecting umbilical cord to at least 120°C. If the flue gas
must be constructed of quartz or borosilicate glass. If an EPA temperature is less than 120°C, then EPA Method 5 configu-
Method 17 (in-stack filtration) sampling configuration is used, ration must be used.
the probe/probe liner may be constructed of borosilicate glass,
7.1.8 Condensing/Absorbing System, consists of eight im-
quartz or, depending on the flue gas temperature, PTFE.
pingers immersed in an ice bath and connected in series with
7.1.3 Pitot Tube, Type S pitot tube. Refer to Section 2.2 of
leak-free ground glass fittings or other non-contaminating
EPA Method 2 for a description.
leak-free fittings. (At no time is silicon grease or other greases
7.1.4 Differential Pressure Gages, inclined manometers or
to be used for this method). The first, second, fourth, fifth,
equivalent devices. Refer to Section 2.1 of EPA Method 2 for
sixth, and eighth impingers are of the Greenburg-Smith design
a description.
modified by replacing the standard tip with a 1.3-cm (0.5-
7.1.5 Filter Holder, constructed of borosilicate glass or
in.)-ID straight glass tube extending to about 1.3 cm (0.5 in.)
PTFE-coated stainless steel with a PTFE filter support or other
from the bottom of the flask. The third and seventh impingers
nonmetallic,non-contaminatingsupport.Donotuseaglassfrit
are also Greenburg-Smith design, but with the standard tip
orstainlesssteelwirescreen.AsiliconerubberorPTFEgasket,
includingtheglassimpingingplate.Thefirst,second,andthird
designed to provide a positive seal against leakage from
impingers contain aqueous 1 N potassium chloride (KCl)
outside or around the filter, may be used.
solution. The fourth impinger contains an aqueous solution of
V V
7.1.6 Connecting Umbilical Tube,heatedPTFEtubing.This
5% / nitric acid (HNO ) and 10% / hydrogen peroxide
V 3 V
tube must be heated to a minimum of 120°C to help prevent
(H O ). The fifth, sixth, and seventh impingers contain an
2 2
W
water and acid condensation. (The umbilical tube is defined as
aqueous solution of 4 % / potassium permanganate
V
V
any tubing longer than 0.5 m that connects the filter holder to
(KMnO ) and 10% / sulfuric acid (H SO ). The last im-
4 V 2 4
the impinger train).
pinger contains silica gel or an equivalent desiccant. Refer to
7.1.7 Probe and Filter Heating System:
Note 1.
7.1.7.1 EPA Method 5 Configuration—For EPA Method 5
NOTE 1—When flue gas streams are sampled with high moisture
configuration, the temperature of the flue gas, sample probe,
content (>20%), additional steps must be taken to eliminate carryover of
and the exit of the sample filter must be monitored using
impinger contents from one sample type to the next. These steps must
temperature sensors capable of measuring temperature to
includeuseofoversizedimpinger(s)oruseofanemptyimpingerbetween
within 3°C (5.4°F). The heating system must be capable of the KCl and HNO –H O . If a dry impinger is used, it must be rinsed as
3 2 2
discussed in 13.2 of this method and the rinse added to the preceding
maintaining the sample gas temperature of the probe and exit
impinger.
of the sample filter to within 615°C (627°F) of the flue gas
temperature.Regardlessofthefluegastemperature,toprevent 7.1.9 Metering System, vacuum gage, leak-free pump, ther-
water and acid condensation, at no time must the probe mometers capable of measuring temperature to within 3°C
temperature,samplefilterexitgastemperature,orthetempera- (5.4°F), and a dry gas meter or controlled orifice capable of
ture of the connecting umbilical cord be less than 120°C. measuring volume to within 2%.
D6784 − 02 (2008)
7.1.10 Barometer, capable of measuring atmospheric pres- 8. Reagents and Materials
sure to within 0.33 kPa (0.1 in. Hg). In many cases, the
8.1 Purity of Reagents—Reagent-grade chemicals shall be
barometric reading may be obtained from a nearby National
used in all tests. Unless otherwise indicated, it is intended that
Weather Service station, in which case, the station value
all reagents conform to the specifications of the Committee on
(which is the absolute barometric pressure) shall be requested.
Analytical Reagents of theAmerican Chemical Society, where
An adjustment for elevation differences between the weather
such specifications are available. Other grades may be used,
stationandsamplingpointshallbeappliedatarateofnegative
provided it is first ascertained that the reagent is of sufficiently
0.33 kPa (0.1 in. Hg) per 30 m (100 ft) elevation increase or
high purity to permit its use without lessening the accuracy of
vice versa for elevation decrease.
the determination.
7.1.11 Gas Density Determination Equipment, temperature
8.2 Purity of Water—Unless otherwise indicated, references
sensorandpressuregage,asdescribedinSection2.3and2.4o
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.