ASTM F3212-16(2023)
(Test Method)Standard Test Method for Coring Testing of Huber Needles
Standard Test Method for Coring Testing of Huber Needles
SIGNIFICANCE AND USE
5.1 This test method determines whether Huber needles are designed and manufactured such that they will not produce a core during simulated implantable port access.
5.2 If a needle produces a core during actual use, leaking of the implantable port may occur. Also, the core may be flushed into the port’s reservoir and subsequently into the patient’s body.
SCOPE
1.1 This test method covers the qualitative measurement of Huber-type needles’ potential to remove septum material during implantable port access (1).2
1.2 This test method does not address other issues that may include, but are not limited to, force measurement during the perforation/withdrawal, septum integrity, and any safety issues.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3212 − 16 (Reapproved 2023)
Standard Test Method for
Coring Testing of Huber Needles
This standard is issued under the fixed designation F3212; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.4 heel, n—the rear cutting edge of the needle bevel.
3.1.5 Huber needle, n—a needle whose tip is angled such
1.1 This test method covers the qualitative measurement of
that the bevel opening is parallel to the main axis of the
Huber-type needles’ potential to remove septum material
cannula. Its special shape slices rather than perforates the
during implantable port access (1).
septum, reducing the chance of leakage due to coring. It is also
1.2 This test method does not address other issues that may
known as a non-coring needed. See Fig. 1.
include, but are not limited to, force measurement during the
3.1.6 implantable port, n—a reservoir placed under the skin
perforation/withdrawal, septum integrity, and any safety issues.
(and usually attached to a catheter) that is made to receive a
1.3 This standard does not purport to address all of the
needle through a septum; it is often used to deliver medication.
safety concerns, if any, associated with its use. It is the
See Fig. 2.
responsibility of the user of this standard to establish appro-
3.1.7 lumen, n—the inside surface of the cannula.
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
3.1.8 septum, n—a feature of an implantable port that allows
1.4 This international standard was developed in accor-
repeated access by a port-access needle, generally composed of
dance with internationally recognized principles on standard-
an elastomeric material. See Fig. 2, Item 1.
ization established in the Decision on Principles for the
3.1.9 stylet, n—a device, preferably metallic, inserted into
Development of International Standards, Guides and Recom-
the lumen to remove a core.
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
4. Summary of Test Method
4.1 A silicone elastomeric disk (surrogate septum or just
2. Referenced Documents
septum thereafter) is clamped into a specifically designed
2.1 ASTM Standards:
septum holder. The test operator accesses the septum with a
D2240 Test Method for Rubber Property—Durometer Hard-
Huber needle in accordance with the needle manufacturer’s
ness
instructions for use, as if the septum was an implantable port.
The lumen at the bevel is examined for the existence of a core,
3. Terminology
preferably before the needle is withdrawn. This is categorized
3.1 Definitions of Terms Specific to This Standard:
as a pass/fail test. Existence of a core in the needle’s cannula is
3.1.1 bevel, n—the slanted part of a needle that creates a
a failed result.
sharp pointed tip.
3.1.2 cannula, n—the tubular part of a needle through which
5. Significance and Use
fluids pass.
5.1 This test method determines whether Huber needles are
3.1.3 core, n—a sliver of septum material that may be
designed and manufactured such that they will not produce a
produced when a needle perforates a septum.
core during simulated implantable port access.
5.2 If a needle produces a core during actual use, leaking of
This test method is under the jurisdiction of ASTM Committee F04 on Medical
the implantable port may occur. Also, the core may be flushed
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
into the port’s reservoir and subsequently into the patient’s
F04.33 on Medical/Surgical Instruments.
Current edition approved Feb. 1, 2023. Published February 2023. Originally body.
approved in 2016. Last previous edition approved in 2016 as F3212 – 16. DOI:
10.1520/F3212-16R23.
6. Apparatus
The boldface numbers in parentheses refer to a list of references at the end of
this standard.
6.1 Clamping Test Fixture, a clamping device which can
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
hold a septum with nominal dimensions of 0.70 in. in diameter
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
and 0.25 in. thick. The clamping device is such that it restrains
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. radial expansion of the septum under axial compression. The
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F3212 − 16 (2023)
FIG. 1 Example of a Huber Needle (partial section through cannula)
FIG. 2 Example of an Implantable Port (section)
compression force is specified when the compression plates are septum is held between two clamps (Fig. 4, Fig. 5, and Fig. 7)
in contact. The distance between the two compressive surfaces with three screws (Figs. 11 and 12). The screws must be
of the fixture plates after the clamping will be 0.213 in. which tightened completely. A polycarbonate cylinder (Fig. 9) holds
results in nominal 15 % compression. See Figs. 3-13. the clamping setup at a height that enables needle penetration
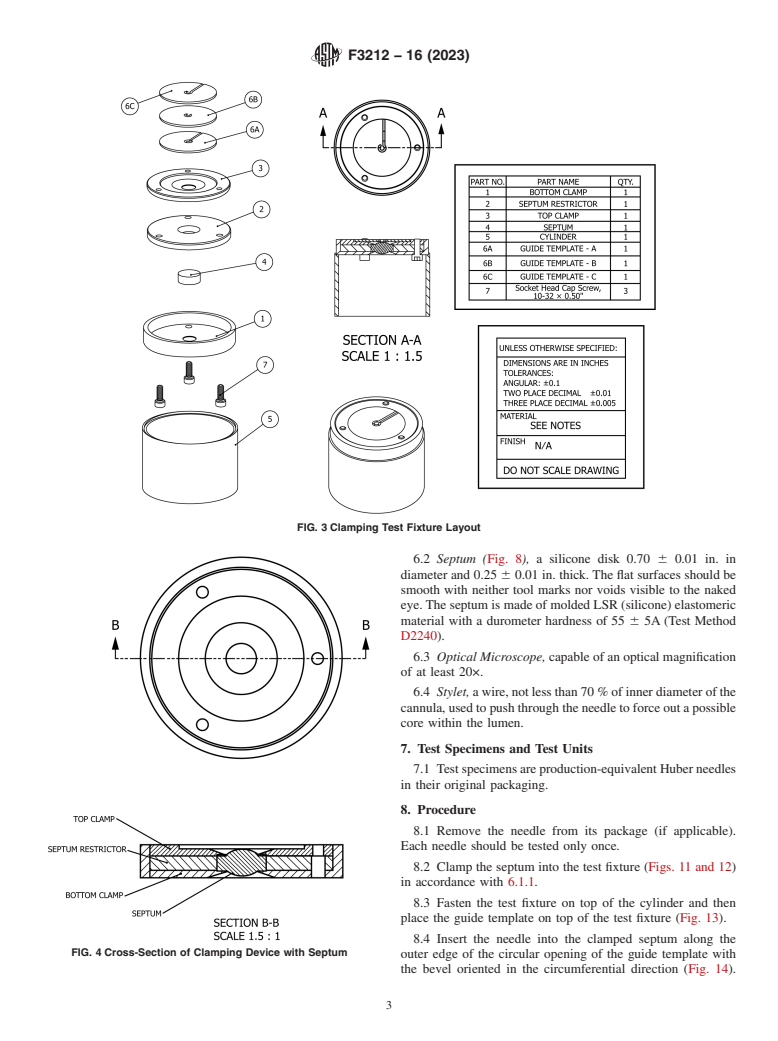
6.1.1 The clamping test fixture consists of six parts (see while protecting the tester from any potential needle sticks.
Figs. 3-13). The septum (Fig. 8) is placed on the opening of the Parts 6a, 6b, and 6c (Fig. 10) are three possible versions of the
septum restrictor (Fig. 6). The septum restrictor with the guide template.
F3212 − 16 (2023)
FIG. 3 Clamping Test Fixture Layout
6.2 Septum (Fig. 8), a silicone disk 0.70 6 0.01 in. in
diameter and 0.25 6 0.01 in. thick. The flat surfaces should be
smooth with neither tool marks nor voids visible to the naked
eye. The septum is made of molded LSR (silicone) elastomeric
material with a durometer hardness of 55 6 5A (Test Method
D2240).
6.3 Optical Microscope, capable of an optical magnification
of at least 20×.
6.4 Stylet, a wire, not less than 70 % of inner diameter of the
cannula, used to push through the needle to force out a possible
core within the lumen.
7. Test Specimens and Test Units
7.1 Test specimens are production-equivalent Huber needles
in their original packaging.
8. Procedure
8.1 Remove the needle from its package (if applicable).
Each needle should be tested only once.
8.2 Clamp the septum into the test fixture (Figs. 11 and 12)
in accordance with 6.1.1.
8.3 Fasten the test fixture on top of the cylinder and then
place the guide template on top of the test fixture (Fig. 13).
8.4 Insert the needle into the clamped septum along the
FIG. 4 Cross-Section of Clamping Device with Septum
outer edge of the circular opening of the guide template with
the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.