ASTM B832-93(2018)
(Guide)Standard Guide for Electroforming with Nickel and Copper
Standard Guide for Electroforming with Nickel and Copper
SIGNIFICANCE AND USE
4.1 The specialized use of the electroplating process for electroforming results in the manufacture of tools and products that are unique and often impossible to make economically by traditional methods of fabrication. Current applications of nickel electroforming include: textile printing screens; components of rocket thrust chambers, nozzles, and motor cases; molds and dies for making automotive arm-rests and instrument panels; stampers for making phonograph records, video-discs, and audio compact discs; mesh products for making porous battery electrodes, filters, and razor screens; and optical parts, bellows, and radar wave guides (1-3).3
4.2 Copper is extensively used for electroforming thin foil for the printed circuit industry. Copper foil is formed continuously by electrodeposition onto rotating drums. Copper is often used as a backing material for electroformed nickel shells and in other applications where its high thermal and electrical conductivities are required. Other metals including gold are electroformed on a smaller scale.
4.3 Electroforming is used whenever the difficulty and cost of producing the object by mechanical means is unusually high; unusual mechanical and physical properties are required in the finished piece; extremely close dimensional tolerances must be held on internal dimensions and on surfaces of irregular contour; very fine reproduction of detail and complex combinations of surface finish are required; and the part cannot be made by other available methods.
SCOPE
1.1 This guide covers electroforming practice and describes the processing of mandrels, the design of electroformed articles, and the use of copper and nickel electroplating solutions for electroforming.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: B832 − 93 (Reapproved 2018)
Standard Guide for
Electroforming with Nickel and Copper
This standard is issued under the fixed designation B832; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope B343 Practice for Preparation of Nickel for Electroplating
with Nickel
1.1 This guide covers electroforming practice and describes
B374 Terminology Relating to Electroplating
the processing of mandrels, the design of electroformed
B489 Practice for Bend Test for Ductility of Electrodepos-
articles, and the use of copper and nickel electroplating
ited and Autocatalytically Deposited Metal Coatings on
solutions for electroforming.
Metals
1.2 This standard does not purport to address all of the
B490 Practice for Micrometer Bend Test for Ductility of
safety concerns, if any, associated with its use. It is the
Electrodeposits
responsibility of the user of this standard to establish appro-
B558 Practice for Preparation of Nickel Alloys for Electro-
priate safety, health, and environmental practices and deter-
plating
mine the applicability of regulatory limitations prior to use.
B571 Practice for Qualitative Adhesion Testing of Metallic
1.3 This international standard was developed in accor-
Coatings
dance with internationally recognized principles on standard-
B578 Test Method for Microhardness of Electroplated Coat-
ization established in the Decision on Principles for the
ings
Development of International Standards, Guides and Recom-
B636 Test Method for Measurement of Internal Stress of
mendations issued by the World Trade Organization Technical
Plated Metallic Coatings with the Spiral Contractometer
Barriers to Trade (TBT) Committee.
B659 Guide for Measuring Thickness of Metallic and Inor-
ganic Coatings
2. Referenced Documents
B849 Specification for Pre-Treatments of Iron or Steel for
2.1 ASTM Standards: Reducing Risk of Hydrogen Embrittlement
B183 Practice for Preparation of Low-Carbon Steel for
E8 Test Methods for Tension Testing of Metallic Materials
Electroplating E384 Test Method for Microindentation Hardness of Mate-
B242 Guide for Preparation of High-Carbon Steel for Elec- rials
troplating
3. Summary of Electroforming Practice
B252 Guide for Preparation of Zinc Alloy Die Castings for
Electroplating and Conversion Coatings 3.1 Electroforming is defined (see Terminology B374)as
B253 Guide for Preparation of Aluminum Alloys for Elec- the production or reproduction of articles by electrodeposition
troplating uponamandrelormoldthatissubsequentlyseparatedfromthe
B254 Practice for Preparation of and Electroplating on deposit.
Stainless Steel
3.2 The basic fabrication steps are as follows: a suitable
B281 Practice for Preparation of Copper and Copper-Base
mandrel is fabricated and prepared for electroplating; the
Alloys for Electroplating and Conversion Coatings
mandrel is placed in an appropriate electroplating solution and
B311 Test Method for Density of Powder Metallurgy (PM)
metal is deposited upon the mandrel by electrolysis; when the
Materials Containing Less Than Two Percent Porosity
required thickness of metal has been applied, the metal-
coveredmandrelisremovedfromthesolution;andthemandrel
is separated from the electrodeposited metal. The electroform
This guide is under the jurisdiction of ASTM Committee B08 on Metallic and
is a separate, free-standing entity composed entirely of elec-
Inorganic Coatings and is the direct responsibility of Subcommittee B08.03 on
trodeposited metal. Electroforming is concerned with the
Engineering Coatings.
fabrication of articles of various kinds.
Current edition approved June 1, 2018. Published June 2018. Originally
approvedin1993.Lastpreviouseditionapprovedin2013asB832 – 93(2013).DOI:
4. Significance and Use
10.1520/B0832-93R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.1 The specialized use of the electroplating process for
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
electroforming results in the manufacture of tools and products
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. that are unique and often impossible to make economically by
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
B832 − 93 (2018)
traditional methods of fabrication. Current applications of the electroform from the mandrel (unless the mandrel is
nickel electroforming include: textile printing screens; compo- removed by melting or chemical dissolution).
nents of rocket thrust chambers, nozzles, and motor cases;
5.1.3 Whether or not a permanent or expendable mandrel
molds and dies for making automotive arm-rests and instru- should be used is largely dependent on the particular article
ment panels; stampers for making phonograph records, video-
that is to be electroformed. If no reentrant shapes or angles are
discs, and audio compact discs; mesh products for making involved, it is possible to use permanent, rigid mandrels that
porous battery electrodes, filters, and razor screens; and optical
can be separated from the finished electroform mechanically
parts, bellows, and radar wave guides (1-3). and reused. If reentrant angles and shapes are involved, it is
necessary to use mandrel materials that can be removed by
4.2 Copper is extensively used for electroforming thin foil
melting or by chemical dissolution, or materials that are
for the printed circuit industry. Copper foil is formed continu-
collapsible, such as polyvinyl chloride and other plastics. In
ouslybyelectrodepositionontorotatingdrums.Copperisoften
some cases, multiple piece mandrels are used that can be
used as a backing material for electroformed nickel shells and
removed even with reentrant features.
in other applications where its high thermal and electrical
5.1.4 Many solid materials can be used to fabricate man-
conductivities are required. Other metals including gold are
drels for electroforming, but the following generalizations may
electroformed on a smaller scale.
help in selecting a suitable material: permanent mandrels are
4.3 Electroforming is used whenever the difficulty and cost
preferred for accuracy and for large production runs; expend-
of producing the object by mechanical means is unusually
able mandrels must be used whenever the part is so designed
high; unusual mechanical and physical properties are required
that a permanent mandrel cannot be withdrawn; and it is
in the finished piece; extremely close dimensional tolerances
important that the mandrel retain its dimensional stability in
must be held on internal dimensions and on surfaces of
warm plating baths. Wax and most plastics expand when
irregular contour; very fine reproduction of detail and complex
exposed to electroplating solutions operated at elevated tem-
combinationsofsurfacefinisharerequired;andthepartcannot
peratures.Insuchcases,itmaybenecessarytouseacidcopper,
be made by other available methods.
nickel sulfamate, and other electroplating solutions that func-
tion at room temperature.
5. Processing of Mandrels for Electroforming
5.2 Mandrel Design:
5.1 General Considerations:
5.2.1 The electroforming operation can often be simplified
5.1.1 Mandrels may be classified as conductors or noncon-
by design changes that do not impair the functioning of the
ductors of electricity, and each of these may be permanent,
piece. Some of the design considerations are summarized in
semipermanent, or expendable (Table 1).
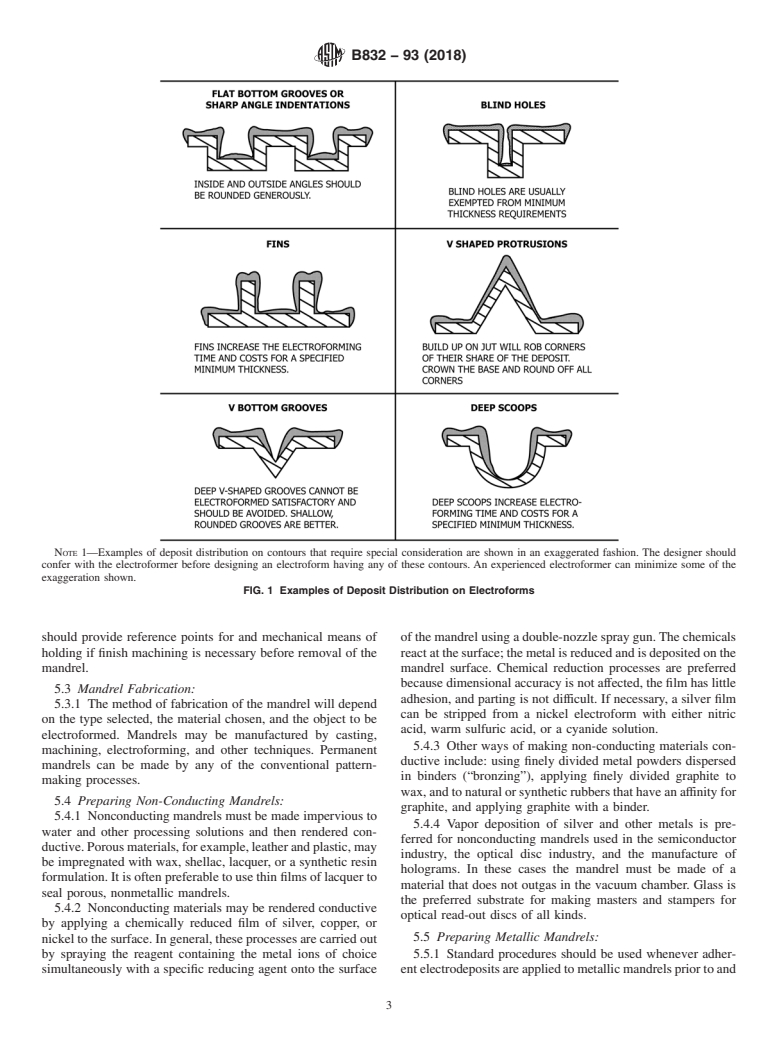
5.2.2, 5.2.3, 5.2.4, 5.2.5, and 5.2.6. Examples of mandrel
shapes that may present problems during electroforming are
TABLE 1 Types of Mandrel Materials
illustrated in Fig. 1.
Types Typical Materials
5.2.2 Exterior (convex) angles should be provided with as
Conductors
generous a radius as possible to avoid excessive build up and
Expendable Low-melting point alloys; for example,
treeing of the deposit during electroforming. Interior (concave)
bismuth-free 92 % tin and 8 % zinc
anglesonthemandrelshouldbeprovidedwithafilletradiusof
Aluminum alloys
at least 0.05 cm per 5 cm (0.02 in. per 2 in.) of length of a side
Zinc alloys
Permanent Nickel
of the angle.
Austenitic Stainless
5.2.3 Whenever possible, permanent mandrels should be
Invar, Kovar
Copper and brass tapered at least 0.08 mm per m (0.001 in. per ft) to facilitate
Nickel-plated steel
removal from the mandrel. (Where this is not permissible, the
Nickel/chromium-plated aluminum
mandrel may be made of a material with a high or low
Nonconductors
coefficient of thermal expansion so that separation can be
effected by heating or cooling).
Expendable Wax
5.2.4 A fine surface finish on the mandrel, achieved by
Glass
Permanent (or Semi-Permanent) Rigid and collapsible plastic; for
lapping or by electropolishing, will generally facilitate separa-
example, epoxy resins and polyvinyl
tion of mandrel and electroform. A finish of 0.05 µm (2 µin.)
chloride
rms is frequently specified.
Wood
5.2.5 Flat bottom grooves, sharp angle indentations, blind
holes, fins, v-shaped projections, v-bottom grooves, deep
5.1.2 Whether or not a mandrel is a conductor will deter-
scoops, slots, concave recesses, and rings and ribs can cause
mine the procedures required to prepare it for electroforming. problems with metal distribution during electroforming unless
Conductive mandrels are usually pure metals or alloys of
inside and outside angles and corners are rounded.
metals and are prepared by standard procedures but may
5.2.6 An engineering drawing of the mandrel, the electro-
require an additional thin parting film to facilitate separation of
formed article, and auxiliary equipment or fixture for separat-
ing the electroform from the mandrel should be prepared. The
drawing of the mandrel should provide for electrical connec-
The boldface numbers in parentheses refer to the list of references at the end of
this standard. tions to be made in nonfunctional areas of the electroform. It
B832 − 93 (2018)
NOTE 1—Examples of deposit distribution on contours that require special consideration are shown in an exaggerated fashion. The designer should
confer with the electroformer before designing an electroform having any of these contours. An experienced electroformer can minimize some of the
exaggeration shown.
FIG. 1 Examples of Deposit Distribution on Electroforms
should provide reference points for and mechanical means of ofthemandrelusingadouble-nozzlespraygun.Thechemicals
holding if finish machining is necessary before removal of the reactatthesurface;themetalisreducedandisdepositedonthe
mandrel. mandrel surface. Chemical reduction processes are preferred
because dimensional accuracy is not affected, the film has little
5.3 Mandrel Fabrication:
adhesion, and parting is not difficult. If necessary, a silver film
5.3.1 The method of fabrication of the mandrel will depend
can be stripped from a nickel electroform with either nitric
on the type selected, the material chosen, and the object to be
acid, warm sulfuric acid, or a cyanide solution.
electroformed. Mandrels may be manufactured by casting,
5.4.3 Other ways of making non-conducting materials con-
machining, electroforming, and other techniques. Permanent
ductive include: using finely divided metal powders dispersed
mandrels can be made by any of the conventional pattern-
in binders (“bronzing”), applying finely divided graphite to
making processes.
wax,andtonaturalorsyntheticrubbersthathaveanaffinityfor
5.4 Preparing Non-Conducting Mandrels:
graphite, and applying graphite with a binder.
5.4.1 Nonconducting mandrels must be made impervious to
5.4.4 Vapor deposition of silver and other metals is pre-
water and other processing solutions and then rendered con-
ferred for nonconducting mandrels used in the semiconductor
ductive.Porousmaterials,forexample,leatherandplastic,may
industry, the optical disc industry, and the manufacture of
be impregnated with wax, shellac, lacquer, or a synthetic resin
holograms. In these cases the mandrel must be made of a
formulation.Itisoftenpreferabletousethinfilmsoflacquerto
material that does not outgas in the vacuum chamber. Glass is
seal porous, nonmetallic mandrels.
the preferred substrate for making masters and stampers for
5.4.2 Nonconducting materials may be rendered conductive
optical read-out discs of all kinds.
by applying a chemically reduced film of silver, copper, or
5.5 Preparing Metallic Mandrels:
nickel to the surface. In general, these processes are carried out
by spraying the reagent containing the metal ions of choice 5.5.1 Standard procedures should be used whenever adher-
simultaneously with a specific reducing agent onto the surface entelectrodepositsareappliedtometallicmandrelspriortoand
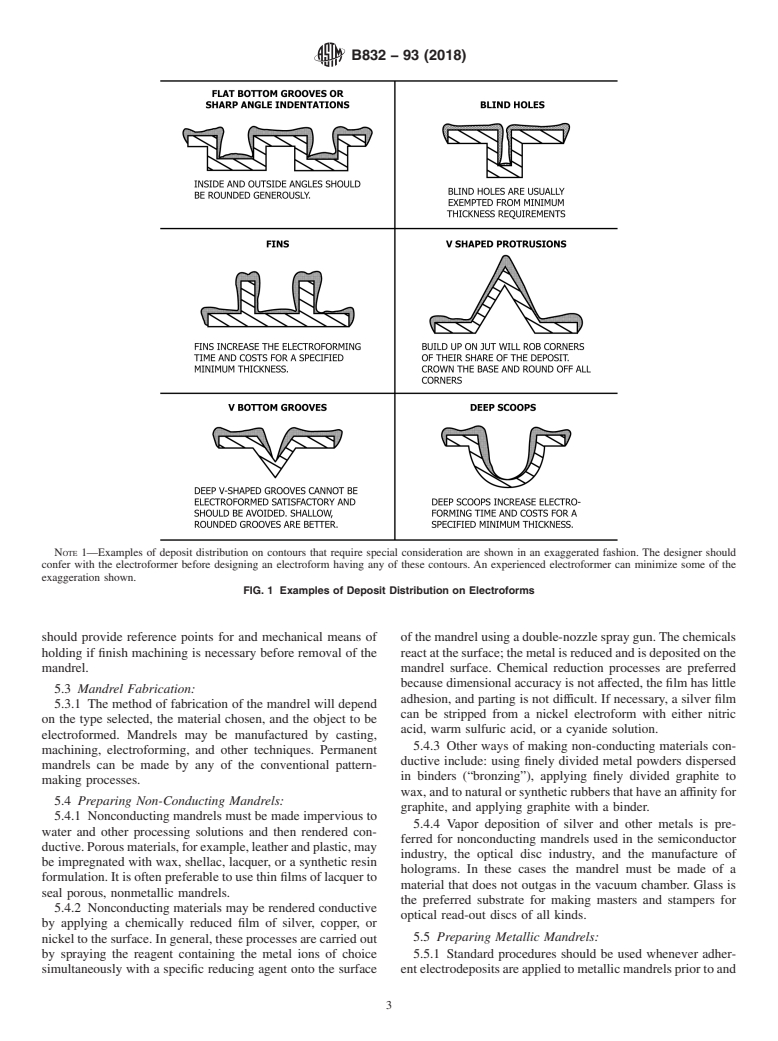
B832 − 93 (2018)
FIG. 1 (continued)
in preparation for electroforming. See Practices B183, B242, 5.5.7 The low-melting point alloys included in Table 1
B254, B281, and B558, for example. employed to make expendable mandrels that can be melted
5.5.2 With most metallic mandrels an additional chemical away have a tendency to leave a residue of tin on the surface
treatment that forms a parting film on the surface is required to oftheelectroform.Themandrelcanbeplatedwithcopperprior
separate the electroform from the mandrel. After removing all to electroforming to prevent this.
traces of grease and oil by means of solvents, various metallic
mandrels are given different treatments for this purpose (see 6. Nickel and Copper Electroforming Solutions
5.5.3, 5.5.4, 5.5.5, 5.5.6, and 5.5.7).
6.1 The choice of metal selected for the electroform will
5.5.3 Stainless steel, nickel, and nickel- or chromium-plated
depend on the mechanical and physical properties required in
steel are cleaned using standard procedures, rinsed, and passi-
the finished article as related to function. The two metals
vatedbyimmersionina2 %solutionofsodiumdichromatefor
selected most frequently are nickel and copper. The operation
30 to 60 s at room temperature. The mandrel must then be
and control of nickel and copper electroforming solutions are
rinsed to remove all traces of the dichromate solution.
described in this section.
5.5.4 Copper and brass mandrels that have been nickel
6.2 The nickel electroplating solutions commonly used for
and/orchromium-platedmaybetreatedasd
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: B832 − 93 (Reapproved 2018)
Standard Guide for
Electroforming with Nickel and Copper
This standard is issued under the fixed designation B832; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope B343 Practice for Preparation of Nickel for Electroplating
with Nickel
1.1 This guide covers electroforming practice and describes
B374 Terminology Relating to Electroplating
the processing of mandrels, the design of electroformed
B489 Practice for Bend Test for Ductility of Electrodepos-
articles, and the use of copper and nickel electroplating
ited and Autocatalytically Deposited Metal Coatings on
solutions for electroforming.
Metals
1.2 This standard does not purport to address all of the
B490 Practice for Micrometer Bend Test for Ductility of
safety concerns, if any, associated with its use. It is the
Electrodeposits
responsibility of the user of this standard to establish appro-
B558 Practice for Preparation of Nickel Alloys for Electro-
priate safety, health, and environmental practices and deter-
plating
mine the applicability of regulatory limitations prior to use.
B571 Practice for Qualitative Adhesion Testing of Metallic
1.3 This international standard was developed in accor-
Coatings
dance with internationally recognized principles on standard-
B578 Test Method for Microhardness of Electroplated Coat-
ization established in the Decision on Principles for the
ings
Development of International Standards, Guides and Recom-
B636 Test Method for Measurement of Internal Stress of
mendations issued by the World Trade Organization Technical
Plated Metallic Coatings with the Spiral Contractometer
Barriers to Trade (TBT) Committee.
B659 Guide for Measuring Thickness of Metallic and Inor-
ganic Coatings
2. Referenced Documents
B849 Specification for Pre-Treatments of Iron or Steel for
2.1 ASTM Standards:
Reducing Risk of Hydrogen Embrittlement
B183 Practice for Preparation of Low-Carbon Steel for E8 Test Methods for Tension Testing of Metallic Materials
Electroplating
E384 Test Method for Microindentation Hardness of Mate-
B242 Guide for Preparation of High-Carbon Steel for Elec- rials
troplating
3. Summary of Electroforming Practice
B252 Guide for Preparation of Zinc Alloy Die Castings for
Electroplating and Conversion Coatings 3.1 Electroforming is defined (see Terminology B374) as
B253 Guide for Preparation of Aluminum Alloys for Elec- the production or reproduction of articles by electrodeposition
troplating upon a mandrel or mold that is subsequently separated from the
B254 Practice for Preparation of and Electroplating on deposit.
Stainless Steel
3.2 The basic fabrication steps are as follows: a suitable
B281 Practice for Preparation of Copper and Copper-Base
mandrel is fabricated and prepared for electroplating; the
Alloys for Electroplating and Conversion Coatings
mandrel is placed in an appropriate electroplating solution and
B311 Test Method for Density of Powder Metallurgy (PM)
metal is deposited upon the mandrel by electrolysis; when the
Materials Containing Less Than Two Percent Porosity
required thickness of metal has been applied, the metal-
covered mandrel is removed from the solution; and the mandrel
is separated from the electrodeposited metal. The electroform
This guide is under the jurisdiction of ASTM Committee B08 on Metallic and
is a separate, free-standing entity composed entirely of elec-
Inorganic Coatings and is the direct responsibility of Subcommittee B08.03 on
trodeposited metal. Electroforming is concerned with the
Engineering Coatings.
fabrication of articles of various kinds.
Current edition approved June 1, 2018. Published June 2018. Originally
approved in 1993. Last previous edition approved in 2013 as B832 – 93(2013). DOI:
4. Significance and Use
10.1520/B0832-93R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.1 The specialized use of the electroplating process for
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
electroforming results in the manufacture of tools and products
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. that are unique and often impossible to make economically by
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
B832 − 93 (2018)
traditional methods of fabrication. Current applications of the electroform from the mandrel (unless the mandrel is
nickel electroforming include: textile printing screens; compo- removed by melting or chemical dissolution).
nents of rocket thrust chambers, nozzles, and motor cases; 5.1.3 Whether or not a permanent or expendable mandrel
molds and dies for making automotive arm-rests and instru-
should be used is largely dependent on the particular article
ment panels; stampers for making phonograph records, video- that is to be electroformed. If no reentrant shapes or angles are
discs, and audio compact discs; mesh products for making
involved, it is possible to use permanent, rigid mandrels that
porous battery electrodes, filters, and razor screens; and optical can be separated from the finished electroform mechanically
parts, bellows, and radar wave guides (1-3).
and reused. If reentrant angles and shapes are involved, it is
necessary to use mandrel materials that can be removed by
4.2 Copper is extensively used for electroforming thin foil
melting or by chemical dissolution, or materials that are
for the printed circuit industry. Copper foil is formed continu-
collapsible, such as polyvinyl chloride and other plastics. In
ously by electrodeposition onto rotating drums. Copper is often
some cases, multiple piece mandrels are used that can be
used as a backing material for electroformed nickel shells and
removed even with reentrant features.
in other applications where its high thermal and electrical
5.1.4 Many solid materials can be used to fabricate man-
conductivities are required. Other metals including gold are
drels for electroforming, but the following generalizations may
electroformed on a smaller scale.
help in selecting a suitable material: permanent mandrels are
4.3 Electroforming is used whenever the difficulty and cost
preferred for accuracy and for large production runs; expend-
of producing the object by mechanical means is unusually
able mandrels must be used whenever the part is so designed
high; unusual mechanical and physical properties are required
that a permanent mandrel cannot be withdrawn; and it is
in the finished piece; extremely close dimensional tolerances
important that the mandrel retain its dimensional stability in
must be held on internal dimensions and on surfaces of
warm plating baths. Wax and most plastics expand when
irregular contour; very fine reproduction of detail and complex
exposed to electroplating solutions operated at elevated tem-
combinations of surface finish are required; and the part cannot
peratures. In such cases, it may be necessary to use acid copper,
be made by other available methods.
nickel sulfamate, and other electroplating solutions that func-
tion at room temperature.
5. Processing of Mandrels for Electroforming
5.2 Mandrel Design:
5.1 General Considerations:
5.2.1 The electroforming operation can often be simplified
5.1.1 Mandrels may be classified as conductors or noncon-
by design changes that do not impair the functioning of the
ductors of electricity, and each of these may be permanent,
piece. Some of the design considerations are summarized in
semipermanent, or expendable (Table 1).
5.2.2, 5.2.3, 5.2.4, 5.2.5, and 5.2.6. Examples of mandrel
shapes that may present problems during electroforming are
TABLE 1 Types of Mandrel Materials
illustrated in Fig. 1.
Types Typical Materials
5.2.2 Exterior (convex) angles should be provided with as
Conductors
generous a radius as possible to avoid excessive build up and
Expendable Low-melting point alloys; for example,
treeing of the deposit during electroforming. Interior (concave)
bismuth-free 92 % tin and 8 % zinc
angles on the mandrel should be provided with a fillet radius of
Aluminum alloys
Zinc alloys at least 0.05 cm per 5 cm (0.02 in. per 2 in.) of length of a side
Permanent Nickel
of the angle.
Austenitic Stainless
5.2.3 Whenever possible, permanent mandrels should be
Invar, Kovar
Copper and brass
tapered at least 0.08 mm per m (0.001 in. per ft) to facilitate
Nickel-plated steel
removal from the mandrel. (Where this is not permissible, the
Nickel/chromium-plated aluminum
mandrel may be made of a material with a high or low
Nonconductors coefficient of thermal expansion so that separation can be
effected by heating or cooling).
Expendable Wax
5.2.4 A fine surface finish on the mandrel, achieved by
Glass
Permanent (or Semi-Permanent) Rigid and collapsible plastic; for
lapping or by electropolishing, will generally facilitate separa-
example, epoxy resins and polyvinyl
tion of mandrel and electroform. A finish of 0.05 µm (2 µin.)
chloride
rms is frequently specified.
Wood
5.2.5 Flat bottom grooves, sharp angle indentations, blind
holes, fins, v-shaped projections, v-bottom grooves, deep
5.1.2 Whether or not a mandrel is a conductor will deter-
scoops, slots, concave recesses, and rings and ribs can cause
mine the procedures required to prepare it for electroforming.
problems with metal distribution during electroforming unless
Conductive mandrels are usually pure metals or alloys of inside and outside angles and corners are rounded.
metals and are prepared by standard procedures but may
5.2.6 An engineering drawing of the mandrel, the electro-
require an additional thin parting film to facilitate separation of
formed article, and auxiliary equipment or fixture for separat-
ing the electroform from the mandrel should be prepared. The
3 drawing of the mandrel should provide for electrical connec-
The boldface numbers in parentheses refer to the list of references at the end of
this standard. tions to be made in nonfunctional areas of the electroform. It
B832 − 93 (2018)
NOTE 1—Examples of deposit distribution on contours that require special consideration are shown in an exaggerated fashion. The designer should
confer with the electroformer before designing an electroform having any of these contours. An experienced electroformer can minimize some of the
exaggeration shown.
FIG. 1 Examples of Deposit Distribution on Electroforms
should provide reference points for and mechanical means of of the mandrel using a double-nozzle spray gun. The chemicals
holding if finish machining is necessary before removal of the react at the surface; the metal is reduced and is deposited on the
mandrel. mandrel surface. Chemical reduction processes are preferred
because dimensional accuracy is not affected, the film has little
5.3 Mandrel Fabrication:
adhesion, and parting is not difficult. If necessary, a silver film
5.3.1 The method of fabrication of the mandrel will depend
can be stripped from a nickel electroform with either nitric
on the type selected, the material chosen, and the object to be
acid, warm sulfuric acid, or a cyanide solution.
electroformed. Mandrels may be manufactured by casting,
5.4.3 Other ways of making non-conducting materials con-
machining, electroforming, and other techniques. Permanent
ductive include: using finely divided metal powders dispersed
mandrels can be made by any of the conventional pattern-
in binders (“bronzing”), applying finely divided graphite to
making processes.
wax, and to natural or synthetic rubbers that have an affinity for
5.4 Preparing Non-Conducting Mandrels:
graphite, and applying graphite with a binder.
5.4.1 Nonconducting mandrels must be made impervious to
5.4.4 Vapor deposition of silver and other metals is pre-
water and other processing solutions and then rendered con-
ferred for nonconducting mandrels used in the semiconductor
ductive. Porous materials, for example, leather and plastic, may
industry, the optical disc industry, and the manufacture of
be impregnated with wax, shellac, lacquer, or a synthetic resin
holograms. In these cases the mandrel must be made of a
formulation. It is often preferable to use thin films of lacquer to
material that does not outgas in the vacuum chamber. Glass is
seal porous, nonmetallic mandrels.
the preferred substrate for making masters and stampers for
5.4.2 Nonconducting materials may be rendered conductive
optical read-out discs of all kinds.
by applying a chemically reduced film of silver, copper, or
5.5 Preparing Metallic Mandrels:
nickel to the surface. In general, these processes are carried out
by spraying the reagent containing the metal ions of choice 5.5.1 Standard procedures should be used whenever adher-
simultaneously with a specific reducing agent onto the surface ent electrodeposits are applied to metallic mandrels prior to and
B832 − 93 (2018)
FIG. 1 (continued)
in preparation for electroforming. See Practices B183, B242, 5.5.7 The low-melting point alloys included in Table 1
B254, B281, and B558, for example. employed to make expendable mandrels that can be melted
5.5.2 With most metallic mandrels an additional chemical away have a tendency to leave a residue of tin on the surface
treatment that forms a parting film on the surface is required to of the electroform. The mandrel can be plated with copper prior
separate the electroform from the mandrel. After removing all to electroforming to prevent this.
traces of grease and oil by means of solvents, various metallic
6. Nickel and Copper Electroforming Solutions
mandrels are given different treatments for this purpose (see
5.5.3, 5.5.4, 5.5.5, 5.5.6, and 5.5.7).
6.1 The choice of metal selected for the electroform will
5.5.3 Stainless steel, nickel, and nickel- or chromium-plated
depend on the mechanical and physical properties required in
steel are cleaned using standard procedures, rinsed, and passi-
the finished article as related to function. The two metals
vated by immersion in a 2 % solution of sodium dichromate for
selected most frequently are nickel and copper. The operation
30 to 60 s at room temperature. The mandrel must then be
and control of nickel and copper electroforming solutions are
rinsed to remove all traces of the dichromate solution.
described in this section.
5.5.4 Copper and brass mandrels that have been nickel
6.2 The nickel electroplating solutions commonly used for
and/or chromiu
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: B832 − 93 (Reapproved 2013) B832 − 93 (Reapproved 2018)
Standard Guide for
Electroforming with Nickel and Copper
This standard is issued under the fixed designation B832; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers electroforming practice and describes the processing of mandrels, the design of electroformed articles,
and the use of copper and nickel electroplating solutions for electroforming.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
B183 Practice for Preparation of Low-Carbon Steel for Electroplating
B242 Guide for Preparation of High-Carbon Steel for Electroplating
B252 Guide for Preparation of Zinc Alloy Die Castings for Electroplating and Conversion Coatings
B253 Guide for Preparation of Aluminum Alloys for Electroplating
B254 Practice for Preparation of and Electroplating on Stainless Steel
B281 Practice for Preparation of Copper and Copper-Base Alloys for Electroplating and Conversion Coatings
B311 Test Method for Density of Powder Metallurgy (PM) Materials Containing Less Than Two Percent Porosity
B343 Practice for Preparation of Nickel for Electroplating with Nickel
B374 Terminology Relating to Electroplating
B489 Practice for Bend Test for Ductility of Electrodeposited and Autocatalytically Deposited Metal Coatings on Metals
B490 Practice for Micrometer Bend Test for Ductility of Electrodeposits
B558 Practice for Preparation of Nickel Alloys for Electroplating
B571 Practice for Qualitative Adhesion Testing of Metallic Coatings
B578 Test Method for Microhardness of Electroplated Coatings
B636 Test Method for Measurement of Internal Stress of Plated Metallic Coatings with the Spiral Contractometer
B659 Guide for Measuring Thickness of Metallic and Inorganic Coatings
B849 Specification for Pre-Treatments of Iron or Steel for Reducing Risk of Hydrogen Embrittlement
E8 Test Methods for Tension Testing of Metallic Materials
E384 Test Method for Microindentation Hardness of Materials
3. Summary of Electroforming Practice
3.1 Electroforming is defined (see Terminology B374) as the production or reproduction of articles by electrodeposition upon
a mandrel or mold that is subsequently separated from the deposit.
3.2 The basic fabrication steps are as follows: a suitable mandrel is fabricated and prepared for electroplating; the mandrel is
placed in an appropriate electroplating solution and metal is deposited upon the mandrel by electrolysis; when the required
thickness of metal has been applied, the metal-covered mandrel is removed from the solution; and the mandrel is separated from
This guide is under the jurisdiction of ASTM Committee B08 on Metallic and Inorganic Coatings and is the direct responsibility of Subcommittee B08.03 on Engineering
Coatings.
Current edition approved Dec. 1, 2013June 1, 2018. Published December 2013June 2018. Originally approved in 1993. Last previous edition approved in 20082013 as
B832 – 93(2008).(2013). DOI: 10.1520/B0832-93R13.10.1520/B0832-93R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
B832 − 93 (2018)
the electrodeposited metal. The electroform is a separate, free-standing entity composed entirely of electrodeposited metal.
Electroforming is concerned with the fabrication of articles of various kinds.
4. Significance and Use
4.1 The specialized use of the electroplating process for electroforming results in the manufacture of tools and products that are
unique and often impossible to make economically by traditional methods of fabrication. Current applications of nickel
electroforming include: textile printing screens; components of rocket thrust chambers, nozzles, and motor cases; molds and dies
for making automotive arm-rests and instrument panels; stampers for making phonograph records, video-discs, and audio compact
discs; mesh products for making porous battery electrodes, filters, and razor screens; and optical parts, bellows, and radar wave
guides (1-3).
4.2 Copper is extensively used for electroforming thin foil for the printed circuit industry. Copper foil is formed continuously
by electrodeposition onto rotating drums. Copper is often used as a backing material for electroformed nickel shells and in other
applications where its high thermal and electrical conductivities are required. Other metals including gold are electroformed on a
smaller scale.
4.3 Electroforming is used whenever the difficulty and cost of producing the object by mechanical means is unusually high;
unusual mechanical and physical properties are required in the finished piece; extremely close dimensional tolerances must be held
on internal dimensions and on surfaces of irregular contour; very fine reproduction of detail and complex combinations of surface
finish are required; and the part cannot be made by other available methods.
5. Processing of Mandrels for Electroforming
5.1 General Considerations:
5.1.1 Mandrels may be classified as conductors or nonconductors of electricity, and each of these may be permanent,
semipermanent, or expendable (Table 1).
TABLE 1 Types of Mandrel Materials
Types Typical Materials
Conductors
Expendable Low-melting point alloys; for example,
bismuth-free 92 % tin and 8 % zinc
Aluminum alloys
Zinc alloys
Permanent Nickel
Austenitic Stainless
Invar, Kovar
Copper and brass
Nickel-plated steel
Nickel/chromium-plated aluminum
Nonconductors
Expendable Wax
Glass
Permanent (or Semi-Permanent) Rigid and collapsible plastic; for
example, epoxy resins and polyvinyl
chloride
Wood
5.1.2 Whether or not a mandrel is a conductor will determine the procedures required to prepare it for electroforming.
Conductive mandrels are usually pure metals or alloys of metals and are prepared by standard procedures but may require an
additional thin parting film to facilitate separation of the electroform from the mandrel (unless the mandrel is removed by melting
or chemical dissolution).
5.1.3 Whether or not a permanent or expendable mandrel should be used is largely dependent on the particular article that is
to be electroformed. If no reentrant shapes or angles are involved, it is possible to use permanent, rigid mandrels that can be
separated from the finished electroform mechanically and reused. If reentrant angles and shapes are involved, it is necessary to use
mandrel materials that can be removed by melting or by chemical dissolution, or materials that are collapsible, such as polyvinyl
chloride and other plastics. In some cases, multiple piece mandrels are used that can be removed even with reentrant features.
The boldface numbers in parentheses refer to the list of references at the end of this standard.
B832 − 93 (2018)
5.1.4 Many solid materials can be used to fabricate mandrels for electroforming, but the following generalizations may help in
selecting a suitable material: permanent mandrels are preferred for accuracy and for large production runs; expendable mandrels
must be used whenever the part is so designed that a permanent mandrel cannot be withdrawn; and it is important that the mandrel
retain its dimensional stability in warm plating baths. Wax and most plastics expand when exposed to electroplating solutions
operated at elevated temperatures. In such cases, it may be necessary to use acid copper, nickel sulfamate, and other electroplating
solutions that function at room temperature.
5.2 Mandrel Design:
5.2.1 The electroforming operation can often be simplified by design changes that do not impair the functioning of the piece.
Some of the design considerations are summarized in 5.2.2, 5.2.3, 5.2.4, 5.2.5, and 5.2.6. Examples of mandrel shapes that may
present problems during electroforming are illustrated in Fig. 1.
5.2.2 Exterior (convex) angles should be provided with as generous a radius as possible to avoid excessive build up and treeing
of the deposit during electroforming. Interior (concave) angles on the mandrel should be provided with a fillet radius of at least
0.05 cm per 5 cm (0.02 in. per 2 in.) of length of a side of the angle.
5.2.3 Whenever possible, permanent mandrels should be tapered at least 0.08 mm per m (0.001 in. per ft) to facilitate removal
from the mandrel. (Where this is not permissible, the mandrel may be made of a material with a high or low coefficient of thermal
expansion so that separation can be effected by heating or cooling).
5.2.4 A fine surface finish on the mandrel, achieved by lapping or by electropolishing, will generally facilitate separation of
mandrel and electroform. A finish of 0.05 μm (2 μin.) rms is frequently specified.
5.2.5 Flat bottom grooves, sharp angle indentations, blind holes, fins, v-shaped projections, v-bottom grooves, deep scoops,
slots, concave recesses, and rings and ribs can cause problems with metal distribution during electroforming unless inside and
outside angles and corners are rounded.
5.2.6 An engineering drawing of the mandrel, the electroformed article, and auxiliary equipment or fixture for separating the
electroform from the mandrel should be prepared. The drawing of the mandrel should provide for electrical connections to be made
NOTE 1—Examples of deposit distribution on contours that require special consideration are shown in an exaggerated fashion. The designer should
confer with the electroformer before designing an electroform having any of these contours. An experienced electroformer can minimize some of the
exaggeration shown.
FIG. 1 Examples of Deposit Distribution on Electroforms
B832 − 93 (2018)
FIG. 1 (continued)
in nonfunctional areas of the electroform. It should provide reference points for and mechanical means of holding if finish
machining is necessary before removal of the mandrel.
5.3 Mandrel Fabrication:
5.3.1 The method of fabrication of the mandrel will depend on the type selected, the material chosen, and the object to be
electroformed. Mandrels may be manufactured by casting, machining, electroforming, and other techniques. Permanent mandrels
can be made by any of the conventional pattern-making processes.
5.4 Preparing Non-Conducting Mandrels:
5.4.1 Nonconducting mandrels must be made impervious to water and other processing solutions and then rendered conductive.
Porous materials, for example, leather and plastic, may be impregnated with wax, shellac, lacquer, or a synthetic resin formulation.
It is often preferable to use thin films of lacquer to seal porous, nonmetallic mandrels.
5.4.2 Nonconducting materials may be rendered conductive by applying a chemically reduced film of silver, copper, or nickel
to the surface. In general, these processes are carried out by spraying the reagent containing the metal ions of choice simultaneously
with a specific reducing agent onto the surface of the mandrel using a double-nozzle spray gun. The chemicals react at the surface;
the metal is reduced and is deposited on the mandrel surface. Chemical reduction processes are preferred because dimensional
accuracy is not affected, the film has little adhesion, and parting is not difficult. If necessary, a silver film can be stripped from a
nickel electroform with either nitric acid, warm sulfuric acid, or a cyanide solution.
5.4.3 Other ways of making non-conducting materials conductive include: using finely divided metal powders dispersed in
binders (“bronzing”), applying finely divided graphite to wax, and to natural or synthetic rubbers that have an affinity for graphite,
and applying graphite with a binder.
5.4.4 Vapor deposition of silver and other metals is preferred for nonconducting mandrels used in the semiconductor industry,
the optical disc industry, and the manufacture of holograms. In these cases the mandrel must be made of a material that does not
outgas in the vacuum chamber. Glass is the preferred substrate for making masters and stampers for optical read-out discs of all
kinds.
5.5 Preparing Metallic Mandrels:
5.5.1 Standard procedures should be used whenever adherent electrodeposits are applied to metallic mandrels prior to and in
preparation for electroforming. See Practices B183, B242, B254, B281, and B558, for example.
5.5.2 With most metallic mandrels an additional chemical treatment that forms a parting film on the surface is required to
separate the electroform from the mandrel. After removing all traces of grease and oil by means of solvents, various metallic
mandrels are given different treatments for this purpose (see 5.5.3, 5.5.4, 5.5.5, 5.5.6, and 5.5.7).
B832 − 93 (2018)
5.5.3 Stainless steel, nickel, and nickel- or chromium-plated steel are cleaned using standard procedures, rinsed, and passivated
by immersion in a 2 % solution of sodium dichromate for 30 to 60 s at room temperature. The mandrel must then be rinsed to
remove all traces of the dichromate solution.
5.5.4 Copper and brass mandrels that have been nickel and/or chromium-plated may be treated as described in 5.5.3. If not
electroplated, the surface can be made passive by immersion in a solution containing 8 g/L sodium sulfide.
5.5.5 Aluminum alloys may require special treatments even when they are used as expendable mandrels to be separated by
chemical dissolution. If the deposits are highly stressed, it may b
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.