ASTM E984-95(2001)
(Guide)Standard Guide for Identifying Chemical Effects and Matrix Effects in Auger Electron Spectroscopy
Standard Guide for Identifying Chemical Effects and Matrix Effects in Auger Electron Spectroscopy
SCOPE
1.1 This guide outlines the types of chemical effects and matrix effects which are observed in Auger electron spectroscopy.
1.2 Guidelines are given for the reporting of chemical and matrix effects in Auger spectra.
1.3 Guidelines are given for utilizing Auger chemical effects for identification or characterization.
1.4 This guide is applicable to both electron excited and X-ray excited Auger electron spectroscopy.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E984–95(Reapproved2001)
Standard Guide for
Identifying Chemical Effects and Matrix Effects in Auger
Electron Spectroscopy
This standard is issued under the fixed designation E 984; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope for a particular element in the specimen under study compared
to the Auger spectrum produced by the same element when it
1.1 This guide outlines the types of chemical effects and
is in some reference form. The differences in the two spectra
matrix effects which are observed in Auger electron spectros-
are said to be due to a “chemical effect” or a “matrix effect.”
copy.
Despite sometimes making elemental identification and quan-
1.2 Guidelines are given for the reporting of chemical and
titative measurements more difficult, these effects in theAuger
matrix effects in Auger spectra.
spectrum are considered valuable tools for characterizing the
1.3 GuidelinesaregivenforutilizingAugerchemicaleffects
environment of the near-surface atoms in a solid.
for identification or characterization.
1.4 This guide is applicable to both electron excited and
5. Defining Auger Chemical Effects and Matrix Effects
X-ray excited Auger electron spectroscopy.
5.1 Ingeneral,Augerchemicalandmatrixeffectsmayresult
1.5 This standard does not purport to address all of the
in(a)ashiftintheenergyofanAugerpeak,(b)achangeinthe
safety concerns, if any, associated with its use. It is the
shape of anAuger electron energy distribution, (c) a change in
responsibility of the user of this standard to establish appro-
the shape of the electron energy loss distribution associated
priate safety and health practices and determine the applica-
with an Auger peak, or (d) a change in the Auger signal
bility of regulatory limitations prior to use.
strengths of an Auger transition. The above changes may be
2. Referenced Documents due to the bonding or chemical environment of the element
(chemical effect) or to the distribution of the element or
2.1 ASTM Standards:
compound within the specimen (matrix effect).
E 673 Terminology Relating to Surface Analysis
5.2 The Auger chemical shift is one of the most commonly
E 827 Practice for Elemental Identification by Auger Elec-
observed chemical effects. A comparison can be made to the
tron Spectroscopy
more familiar chemical shifts in XPS (X-ray photoelectron
E 983 Guide for Minimizing Unwanted Electron Beam
spectroscopy) photoelectron lines, where energy shifts are
Effects In Auger Electron Spectroscopy
caused by changes in the ionic charge on an atom, the lattice
E 996 Practice for Reporting Data in Auger Electron Spec-
potential at that atomic site, and the final-state relaxation
troscopy
energy contributed by adjacent atoms (1 and 2). Coverage by
3. Terminology
gas adsorbates on metal surfaces may also cause shifts in the
metal Auger peak energies (3). The magnitude of the Auger
3.1 Terms used in Auger electron spectroscopy are defined
chemical shift will usually be different from the XPS photo-
in Terminology E 673.
electron shift because the Auger process involves a two-hole
4. Significance and Use
final state for the atom which is more strongly influenced by
extra-atomic relaxation. Frequently an Auger chemical shift is
4.1 Auger electron spectroscopy is often capable of yielding
larger than an XPS chemical shift (see Fig. 1).
informationconcerningthechemicalandphysicalenvironment
5.2.1 Related to chemical shifts is the (modified) Auger
of atoms in the near-surface region of a solid as well as giving
parameter, defined as the sum of the photoelectron binding
elemental and quantitative information. This information is
energy and the Auger electron kinetic energy (4). Because the
manifestedaschangesintheobservedAugerelectronspectrum
Auger parameter is the difference between two line energies of
the same element of the same specimen, it is independent of
any electrical charging of the specimen and spectrometer
This guide is under the jurisdiction of ASTM Committee E-42 on Surface
energy reference level, making it easier to identify chemical
Analysis and is the direct responsibility of Subcommittee E42.03 onAuger Electron
Spectroscopy and XPS.
Current edition approved Sept. 10, 1995. Published November 1995. Originally
published as E 984 – 84. Last previous edition E 984 – 89. The boldface numbers in parentheses refer to the references at the end of this
Annual Book of ASTM Standards, Vol 03.06. standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E984–95 (2001)
FIG. 1 Comparison of X-ray Excited Cd MNN Auger and 3d
Photoelectron Energy Shifts for Cd Metal, CdO, and CdF (Ref 13)
FIG. 2 Carbon KLLAuger Spectra for Mo C, SiC, Graphite, and
states of elements in insulating specimens. Naturally, since
Diamond (Ref 14)
bothphotoelectronlinesandAugerlinesmustbemeasured,the
Auger parameter can only be used with X-ray excited spectra.
5.3 The second category of chemical information from
Auger spectroscopy is the Auger lineshapes observed for
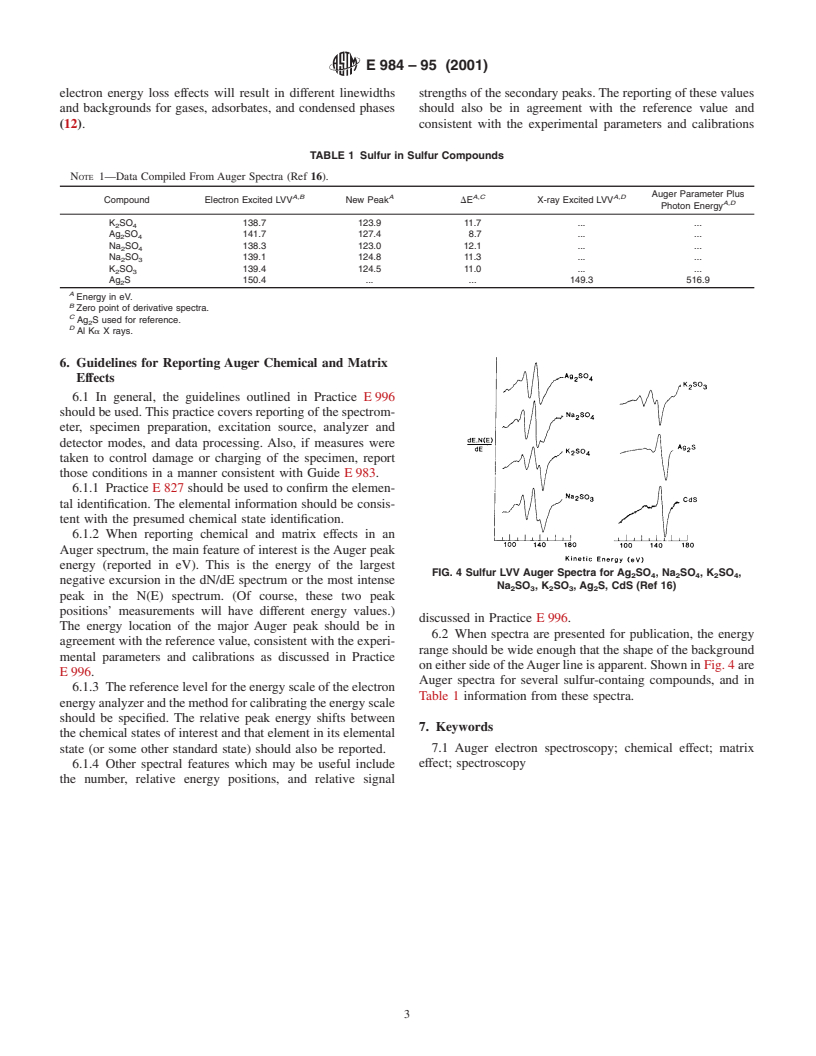
transitions involving valence electron orbitals. Shown in Fig. 2
and Fig. 3 are selected lineshapes for carbon KLL and
aluminum LVV Auger transitions for different chemical states
of those elements. While it is possible to relate the prominent
peaks in the Auger spectrum to transitions from particular
bands in the density of states (for solids) or to particular
molecular orbitals (for molecules) (5), this is not an easy task.
The large number of possible two-hole final states, taken
together with shake-up and shake-off transitions and uncer-
tainty on all their final energies and intensities make the job of
constructing a valence orbital density map from the Auger
spectrum next to impossible for all but the simplest systems.
Further, some spectra exhibit a quasiatomic character (6).
Accordingly, most studies use the “fingerprint” approach when
attempting to identify unknown species based on their Auger
(a) Almost no Oxidation (b) Partial Oxidation (c) After Oxidation has
Reached a Satura-
lineshape. Of course reference spectra are necessary in this
tion Stage
approach for a positive identification.
FIG. 3 Changes in the Aluminum LVV Auger spectrum as
5.4 Other effects besides energy shifts and valence line-
Oxygen is Absorbed on the Surface (Ref 15)
shapes may be classified as chemical effects inAuger spectros-
copy. For instance, many body effects in metals, such as
plasmons, may make the lineshapes of Auger transitions of
(8). Relative intensities of several Auger transitions may
atoms in the metallic state very different from the Auger
change, either from attenuation of overlayers (9), or from
lineshapes for other chemical states, even for transitions
involving only core-type electrons, Al and Mg (7). In single different chemical states r
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.