ASTM E2210-06
(Specification)Standard Specification for Guideline Elements Model version 2 (GEM II)-Document Model for Clinical Practice Guidelines
Standard Specification for Guideline Elements Model version 2 (GEM II)-Document Model for Clinical Practice Guidelines
ABSTRACT
This specification updates a standard representation for storing and organizing the heterogeneous information contained in clinical practice guidelines, intended to facilitate translation of natural-language guideline documents into a format that can be processed by computers. This specification is based on the guideline elements model version 2 (GEM II) created at the Yale Center for Medical Informatics by health services researchers and informatics specialists, and designed to serve as a comprehensive XML-based guideline document representation.
SCOPE
1.1 This specification updates a standard representation for storing and organizing the heterogeneous information contained in clinical practice guidelines. This specification is intended to facilitate translation of natural-language guideline documents into a format that can be processed by computers. It can be used to represent document content throughout the entire guideline life cycle. Information at both high and low levels of abstraction can be accommodated. This specification is based on the guideline elements model (GEM) created at the Yale Center for Medical Informatics and designed to serve as a comprehensive XML-based guideline document representation.
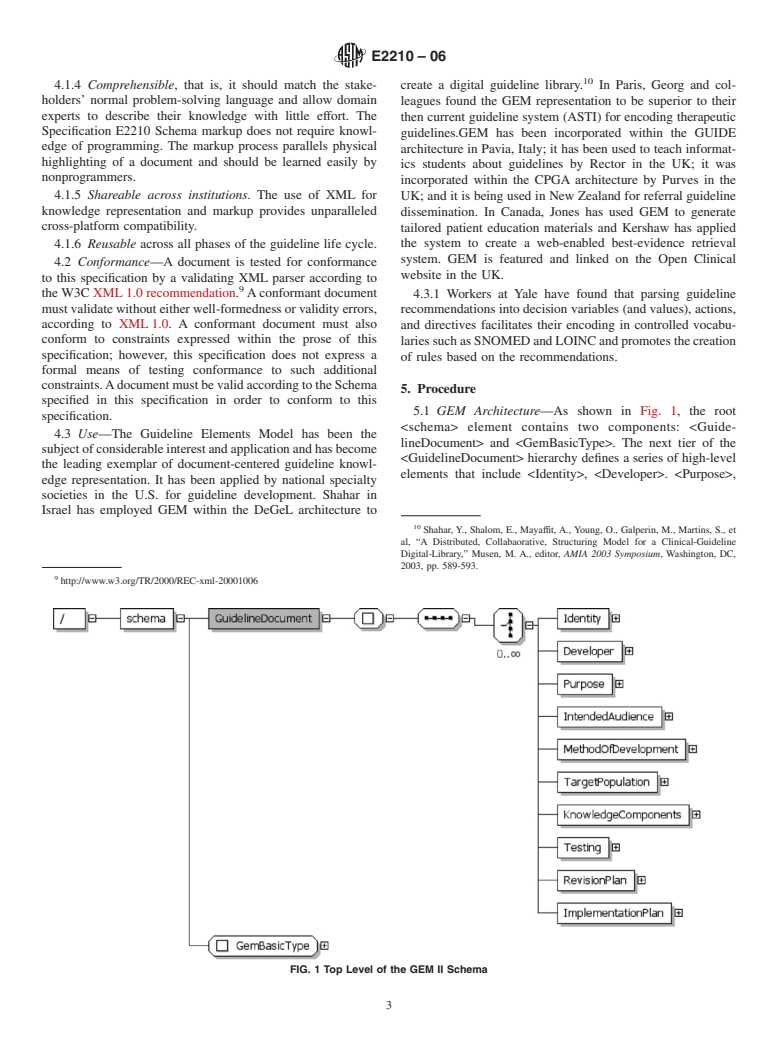
1.2 This specification refers to and makes use of recommendations from the World Wide Web consortium, the W3C.
1.3 Standard Guideline Schema This specification defines a standard Schema for clinical practice guidelines. The Schema is included in Annex A1.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory requirements prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: E2210 – 06
Standard Specification for
Guideline Elements Model version 2 (GEM II)—Document
1
Model for Clinical Practice Guidelines
This standard is issued under the fixed designation E2210; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* platform-independent data access via XML, or for marking up
a given type of SGML document.
1.1 This specification updates a standard representation for
3.1.2 extensible markup language (XML)—standard from
storing and organizing the heterogeneous information con-
the World Wide Web Consortium (W3C) that provides for
tained in clinical practice guidelines. This specification is
tagging of information content within documents, offering a
intended to facilitate translation of natural-language guideline
means of representation of content in a format that is both
documents into a formatthatcanbeprocessedbycomputers.It
humanandmachinereadable.Throughtheuseofcustomizable
can be used to represent document content throughout the
style sheets and schemas, information can be represented in a
entire guideline life cycle. Information at both high and low
uniform way, allowing for interchange of both content (data)
levels of abstraction can be accommodated. This specification
and format (metadata).
is based on the guideline elements model (GEM) created at the
3.1.3 health level 7 (HL7)—a standards organization tradi-
Yale Center for Medical Informatics and designed to serve as
tionally focused on standards for healthcare information inter-
a comprehensive XML-based guideline document representa-
change. HL7 messages are the dominant standard for peer-to-
tion.
peer exchange of clinical text-based information. More
1.2 Thisspecificationreferstoandmakesuseofrecommen-
2
recently, HL7 has developed a comprehensive object model of
dations from the World Wide Web consortium, the W3C.
the healthcare enterprise and the first level of an XML clinical
1.3 Standard Guideline Schema—This specification defines
document architecture.
astandardSchemaforclinicalpracticeguidelines.TheSchema
3.1.4 HL7 clinical document architecture (CDA)—a docu-
is included in Annex A1.
ment markup standard for the structure and semantics of
1.4 This standard does not purport to address all of the
exchanged clinical documents. A clinical document is a docu-
safety concerns, if any, associated with its use. It is the
mentation of observations and other services with the follow-
responsibility of the user of this standard to establish appro-
ing characteristics: persistence, stewardship, potential for au-
priate safety and health practices and determine the applica-
thentication, wholeness, and human readability. A CDA
bility of regulatory requirements prior to use.
documentisadefinedandcompleteinformationobjectthatcan
2. Referenced Documents exist outside of a message and can include text, sounds, and
other multimedia content.
2.1 W3C World Wide Web Consortium:
3
3.1.5 hypertext markup language (HTML)—the language
XML 1.0 Recommendation
4
used in creating a web page. Its origin is an implementation of
XML Schema 1.0
SGML DTD. It provides tags regarding the way a document
3. Terminology
should be displayed in the text of an HTML document, which
act as commands that a browser interprets when downloading
3.1 Definitions:
an HTML file.
3.1.1 document type definition (DTD)—the formal defini-
3.1.6 namespaces—provide a simple method for qualifying
tion of the elements, structures, and rules for enabling
element and attribute names used in XML documents. This is
accomplished by associating a particular tag set by associating
a prefix with a URI reference. XML namespaces provides a
1
This specification is under the jurisdiction of ASTM Committee E31 on
mechanism for authoring compound documents (documents
Healthcare Informatics and is the direct responsibility of Subcommittee E31.35 on
consisting of elements and attributes from multiple DTDs or
Healthcare Data Analysis.
schemas) in such a way that will provide global identification
Current edition approved Dec. 1, 2006. Published January 2007. Originally
approved in 2002. Last previous edition approved in 2002 as E2210 – 02. DOI:
without collisions of names that are the same but are used
10.1520/E2210-06.
differently.
2
http://www.w3.org
3 3.1.7 parser—a specialized software program that recog-
http://www.w3.org/XML/
4
http://www.w3.org/XML/Schema nizesmarkupinadocumentanddifferentiatesthecontentfrom
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, We
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.