ASTM D1607-91(2000)e1
(Test Method)Standard Test Method for Nitrogen Dioxide Content of the Atmosphere (Griess-Saltzman Reaction)
Standard Test Method for Nitrogen Dioxide Content of the Atmosphere (Griess-Saltzman Reaction)
SCOPE
1.1 This test method covers the manual determination of nitrogen dioxide (NO2) in the atmosphere in the range from 4 to 10 000 ug/m3 (0.002 to 5 ppm(v)) when sampling is conducted in fritted-tip bubblers.
1.2 For concentrations of NO2 in excess of 10 mg/m3 (5 ppm(v)), as occur in industrial atmospheres, gas burner stacks, or automotive exhaust, or for samples relatively high in sulfur dioxide content, other methods should be applied. See for example Test Method D1608.
1.3 The maximum sampling period is 60 min at a flow rate of 0.4 L/min.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See also 7.2.2 for other precautions.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation:D1607–91(Reapproved 2000)
Standard Test Method for
Nitrogen Dioxide Content of the Atmosphere (Griess-
Saltzman Reaction)
This standard is issued under the fixed designation D 1607; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
e NOTE—Editorial corrections were made throughout in September 2000.
1. Scope D 1357 Practice for Planning the Sampling of the Ambient
2 Atmosphere
1.1 This test method covers the manual determination of
D 1608 Test Method for Oxides of Nitrogen in Gaseous
nitrogen dioxide (NO ) in the atmosphere in the range from 4
Combustion Products (Phenol-Disulfonic Acid Proce-
to 10 000 µg/m (0.002 to 5 ppm(v)) when sampling is
dure)
conducted in fritted-tip bubblers.
D 3195 Practice for Rotameter Calibration
1.2 For concentrations of NO in excess of 10 mg/m (5
D 3609 Practice for Calibration Techniques Using Perme-
ppm(v)), as occur in industrial atmospheres, gas burner stacks,
ation Tubes
or automotive exhaust, or for samples relatively high in sulfur
D 3631 Test Methods for Measuring Surface Atmospheric
dioxide content, other methods should be applied. See for
Pressure
example Test Method D 1608.
E 1 Specification for ASTM Thermometers
1.3 The maximum sampling period is 60 min at a flow rate
E 128 Test Method for Maximum Pore Diameter and Per-
of 0.4 L/min.
meability of Rigid Porous Filters for Laboratory Use
1.4 The values stated in SI units are to be regarded as the
standard. The values given in parentheses are for information
3. Terminology
only.
3.1 For definitions of terms used in this test method, refer to
1.5 This standard does not purport to address all of the
Terminology D 1356.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Summary of Test Method
priate safety and health practices and determine the applica-
4.1 The NO is absorbed in an azo-dye-forming reagent
bility of regulatory limitations prior to use. See also 7.2.2 for
(1). Ared-violet color is produced within 15 min, the intensity
other precautions.
of which is measured spectrophotometrically at 550 nm.
2. Referenced Documents
5. Significance and Use
2.1 ASTM Standards:
5.1 Nitrogendioxideplaysanimportantroleinphotochemi-
D 1071 Test Methods for Volumetric Measurement of Gas-
cal smog-forming reactions and, in sufficient concentrations, is
eous Fuel Samples
4 deleterious to health, agriculture, materials, and visibility.
D 1193 Specification for Reagent Water
5.2 In combustion processes, significant amounts of nitric
D 1356 Terminology Relating to Sampling and Analysis of
5 oxide (NO) may be produced by combination of atmospheric
Atmospheres
nitrogen and oxygen; at ambient temperatures NO can be
converted to NO by oxygen and other atmospheric oxidants.
Nitrogendioxidemayalsobegeneratedfromprocessesinvolv-
This test method is under the jurisdiction of ASTM Committee D22 on
Sampling andAnalysis ofAtmospheres, and is the direct responsibility of Subcom-
ing nitric acid, nitrates, the use of explosives, and welding.
mittee D 22.03 on Ambient Atmospheres and Source Emissions.
Current edition approved Aug. 15, 1991. Published December 1991. Originally
{1
published as D 1607 – 58. Last previous edition D 1607 – 91 (1995) .
Adapted from “Selected Methods for the Measurement ofAir Pollutants,” PHS
Publication No 999-AP-11, May 1965. A similar version has been submitted to the
Intersociety Committee. Annual Book of ASTM Standards, Vol 14.03.
3 7
Annual Book of ASTM Standards, Vol 05.05. Annual Book of ASTM Standards, Vol 14.02.
4 8
Annual Book of ASTM Standards, Vol 11.01. The boldface numbers in parentheses refer to the list of references appended to
Annual Book of ASTM Standards, Vol 11.03. this method.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
D1607–91 (2000)
6. Interferences discolored, carefully clean with concentrated chromic-sulfuric
acid mixture, and rinse well with water and redetermine the
6.1 Aten-fold ratio of sulfur dioxide (SO )toNO produces
2 2
maximum pore diameter.
no effect. A thirty-fold ratio slowly bleaches the color to a
slight extent. The addition of acetone to the reagent retards the
NOTE 1—Caution: Do not dispose of this reagent in the drain system.
fadingbyformingatemporaryadditionproductwithSO .This
7.2.3 Rinse the bubbler thoroughly with water and allow to
permits reading the color intensity within 4 to 5 h (instead of
dry before using.
the 45 min required without the acetone) without appreciable
7.3 Mist Eliminator or Gas Drying Tube, filled with acti-
losses.
vated charcoal or soda lime is used to prevent damage to the
6.2 A five-fold ratio of ozone to NO will cause a small
flowmeter and pump.
interference, the maximal effect occurring in 3 h. The reagent
7.4 Air-Metering Device—Acalibrated, glass, variable-area
assumes a slightly orange tint.
flowmeter, or dry gas meter coupled with a flow indicator
6.3 Peroxyacetyl nitrate (PAN) can produce a color change
capable of accurately measuring a flow of 0.4 L/min.
in the absorbing reagent. However, in ordinary ambient air, the
7.5 Thermometer—ASTM Thermometer 33C, meeting the
concentration of PAN is too low to cause any significant error
requirements of Specification E 1, will be suitable for most
in the measurement of NO .
applications of this test method.
6.4 Interferences may exist from other nitrogen oxides and
7.6 Manometer, accurate to 670 Pa (0.20 in. Hg). See Test
other gases that might be found in polluted air.
Methods D 3631.
7.7 Air Pump—A suction pump capable of drawing the
7. Apparatus
required sample flow for intervals of up to 60 min is suitable.
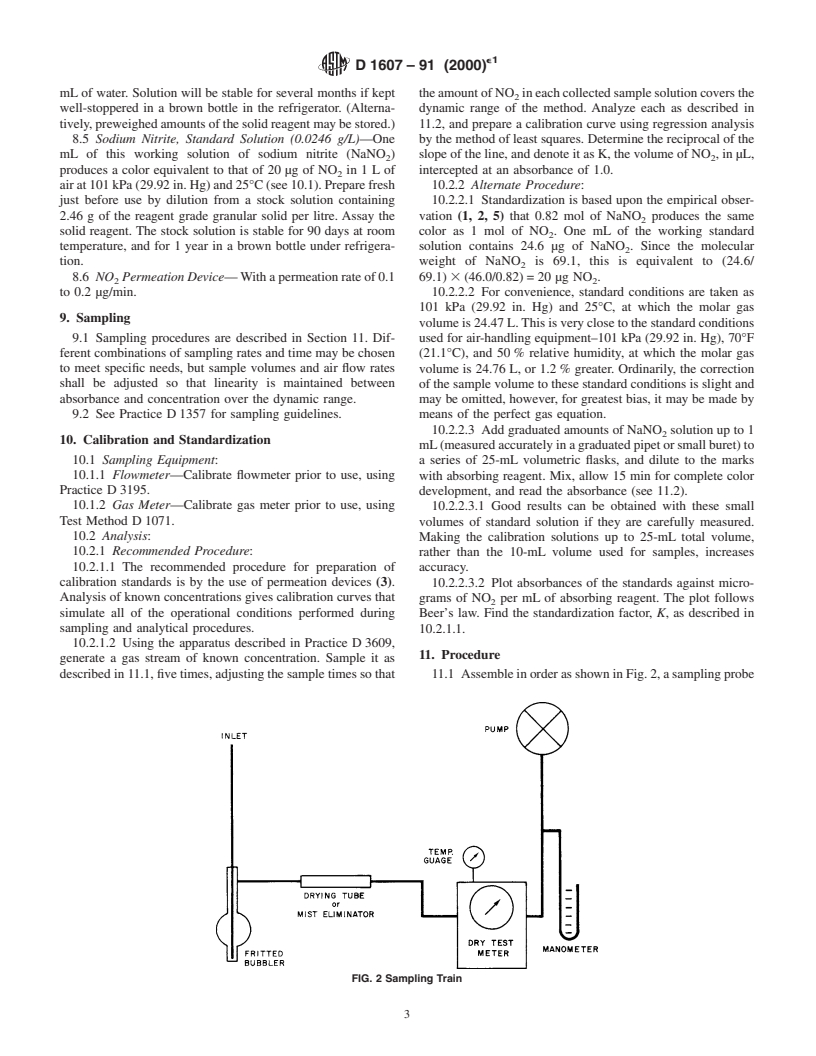
7.1 Sampling Probe—A glass or TFE-fluorocarbon (pre-
7.8 Spectrophotometer or Colorimeter— An instrument
ferred) tube, 6 to 10 mm in diameter provided with a
suitable for measuring the intensity of absorption at 550 nm,
downwindfacingintake(funnelortip).Thedeadvolumeofthe
with stoppered tubes or cuvettes. The wavelength band-width
system should be kept minimal to avoid losses of NO on the
is not critical for this determination.
surfaces of the apparatus.
7.9 Stopwatch or Timer.
7.2 Absorber—An all-glass bubbler with a 60-µm maxi-
mum pore diameter frit, similar to that illustrated in Fig. 1.
8. Reagents and Materials
7.2.1 The porosity of the fritted bubbler, as well as the
8.1 Reagent grade chemicals shall be used in all tests. All
sampling flow rate, affect absorption efficiency. An efficiency
reagents shall conform to the specifications of the Committee
of over 95 % may be expected with a flow rate of 0.4 L/min or
on Analytical Reagents of the American Chemical Society,
less and a maximum pore diameter of 60 µm. Frits having a
where such specifications are available. Other grades may be
maximum pore diameter less than 60 µm will have a higher
used, provided it is first ascertained that the reagent is of
efficiency but will require an inconvenient pressure drop for
sufficiently high purity to permit its use without lessening the
sampling. Considerably lower efficiencies are obtained with
accuracy of the determination.
coarser frits.
8.2 Purity of Water—Unless otherwise indicated, water
7.2.2 Measure the porosity of an absorber in accordance
shall be deionized water in accordance with Specification
with Test Method E 128. If the frit is clogged or visibly
D 1193 for Type I or II reagent water. Water shall be free of
nitrite.
8.3 Absorbing Reagent—Dissolve5gof anhydrous sulfa-
nilic acid (or 5.5 g of sulfanilic acid monohydrate) in almost a
L of water containing 140 mL of glacial acetic acid. Gentle
heating is permissible to speed up the process. To the cooled
mixture, add 20 mL of the 0.1 % stock solution of N-(1-
naphthyl)-ethylenediamine dihydrochloride, and 10 mL of
acetone. Dilute to 1 L. The solution will be stable for several
months if kept well-stoppered in a brown bottle in the
refrigerator. The absorbing reagent shall be at room tempera-
ture before use. Avoid lengthy contact with air during prepa-
rationandusesincediscolorationofreagentwillresultbecause
of absorption of NO .
8.4 N-(1-Naphthyl)-Ethylenediamine Dihydrochloride,
Stock Solution (0.1 %)—Dissolve 0.1 g of the reagent in 100
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
FIG. 1 Fritted Bubbler for Sampling Nitrogen Dioxide MD.
e1
D1607–91 (2000)
mL of water. Solution will be stable for several months if kept theamountofNO ineachcollectedsamplesolutioncoversthe
well-stoppered in a brown bottle in the refrigerator. (Alterna- dynamic range of the method. Analyze each as described in
tively,preweighedamountsofthesolidreagentmaybestored.) 11.2, and prepare a calibration curve using regression analysis
8.5 Sodium Nitrite, Standard Solution (0.0246 g/L)—One by the method of least squares. Determine the reciprocal of the
mL of this working solution of sodium nitrite (NaNO ) slope of the line, and denote it as K, the volume of NO ,inµL,
2 2
produces a color equivalent to that of 20 µg of NO in1Lof intercepted at an absorbance of 1.0.
airat101kPa(29.92in.Hg)and25°C(see10.1).Preparefresh 10.2.2 Alternate Procedure:
just before use by dilution from a stock solution containing 10.2.2.1 Standardization is based upon the e
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.