ASTM F2638-07
(Test Method)Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
SCOPE
1.1 This test method measures the aerosol filtration performance of porous packaging materials by creating a defined aerosol of 1.0 m particles and assessing the filtration efficiency of the material using either single or dual particle counters.
1.2 This test method is applicable to porous materials used to package terminally sterilized medical devices.
1.3 The intent of this test method is to determine the flow rate through a material at which maximum penetration occurs. The porous nature of some materials used in sterile packaging applications might preclude evaluation by means of this test method. The maximum penetration point of a particular material could occur at a flow rate that exceeds the flow capacity of the test apparatus. As such, this test method may not be useful for evaluating the maximum penetration point of materials with a Bendtsen flow rate above 4000 mL/min as measured by ISO 5636-3.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
Designation: F2638 – 07
Standard Test Method for
Using Aerosol Filtration for Measuring the Performance of
Porous Packaging Materials as a Surrogate Microbial
Barrier
This standard is issued under the fixed designation F2638; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 This test method measures the aerosol filtration perfor- 3.1 Definitions:
mance of porous packaging materials by creating a defined 3.1.1 challenge aerosol—asufficientquantityofaerosolized
aerosol of 1.0 µm particles and assessing the filtration effi- 1.0 µm particles that enable effective particle counting in the
ciency of the material using either single or dual particle filtrate aerosol.
counters. 3.1.2 filtrate aerosol—particles that remain aerosolized af-
1.2 This test method is applicable to porous materials used ter passage through the test specimen.
to package terminally sterilized medical devices. 3.1.3 maximum penetration—the highest percent concentra-
1.3 The intent of this test method is to determine the flow tionofparticlesinthefiltrateaerosolwhenaspecimenistested
rate through a material at which maximum penetration occurs. over a range of pressure differentials or air flow rates.
The porous nature of some materials used in sterile packaging 3.2 Abbreviations and Symbols:
applications might preclude evaluation by means of this test
Symbol Unit Description
C n Average particle count of the challenge aerosol
S
method. The maximum penetration point of a particular mate-
when using a single particle counter (Method A).
rial could occur at a flow rate that exceeds the flow capacity of
C n Average particle count of the filtrate aerosol.
F
the test apparatus.As such, this test method may not be useful C n Average particle count of the challenge aerosol.
C
C N Average particle count of the filtrate aerosol prior to
LR
forevaluatingthemaximumpenetrationpointofmaterialswith
correction for dilution.
a Bendtsen flow rate above 4000 mL/min as measured by
R % Percentage of particles from the challenge aerosol
ISO 5636–3. that remain in the filtrate aerosol.
R % The calculated maximum of R.
M
1.4 The values stated in SI units are to be regarded as the
P cm WC Pressure differential across a test specimen due to
standard. The values given in parentheses are for information
the air flow required by the particle counter.
only.
P cm WC Pressure differential across a test specimen.
F L/m/cm Air flow rate through the test specimen.
1.5 This standard does not purport to address all of the
F L/m/cm Air flow rate required by the particle counter when
safety concerns, if any, associated with its use. It is the
measuring the filtrate aerosol.
responsibility of the user of this standard to establish appro- F L/m/cm Air flow rate at which maximum penetration occurs.
M
priate safety and health practices and determine the applica-
4. Safety
bility of regulatory limitations prior to use.
4.1 The waste and the vacuum venturi vents for the test
2. Referenced Documents
equipment described in this test method emit an aerosol of
polystyrene particles and salt residues. These aerosols should
2.1 ISO Standard:
be exhausted from any enclosed environment or collected and
ISO 5636–3 Paper and Board—Determination of Air Per-
filtered to remove all particles.
meance (Medium Range)—Part 3: Bendtsen Method
5. Summary of Test Method
1 5.1 A porous packaging material test specimen is placed in
This test method is under the jurisdiction ofASTM Committee F02 on Flexible
a sample holder in such a way as to create a filter between the
Barrier Packaging and is the direct responsibility of Subcommittee F02.15 on
Chemical/ Safety Properties.
challenge and filtrate aerosols. On the challenge side of the
Current edition approved Aug. 1, 2007. Published September 2007. DOI:
sample holder, an aerosol of particles is presented to the
10.1520/F2638-07.
surface of the test specimen. An air flow is generated through
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036, http://www.ansi.org. the test specimen. A laser particle counter is used to monitor
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
F2638 – 07
NOTE—The point of maximum penetration is indicated by the upward pointing triangle.
FIG. 1 A Typical Curve Showing Penetration as a Function of Flow Rate
the particle concentrations in the challenge and filtrate aero- filtrate aerosol over a time period of not less than 45 s,
sols. Particle concentrations will be measured over a range of beginning no sooner than 1 min after a change in flow rate.
flow rates in order to measure the percent penetration over the 5.3 At the pressures used in this test, pressure differential
range of flow rates and determine the point of maximum across the sample and flow rate through the material are
penetration. directly proportional. Pressure will be varied over a range that
5.2 This test uses an aerosol of polystyrene latex particles will ideally have at least two measurements at flow rates that
(PSL) with a geometric mean particle diameter of 1.0 µm and are higher and lower than the flow rate that demonstrates the
a standard deviation of less than 0.05 µm. maximum penetration.
5.2.1 A single particle counter may be used to sequentially 5.4 The reported results are the maximum penetration and
measure the challenge and filtrate aerosols or two particle the flow rate at which it occurs.
counters may be used to measure them continuously. When
6. Significance and Use
using a single particle counter the challenge and filtrate
aerosols will be sequentially measured for each test flow rate. 6.1 This test method has been developed as a result of
research performed by Air Dispersion Limited (Manchester,
The filtrate aerosol concentration is reported as the average
concentration of the filtrate aerosol over a time period of 45 to UK) and funded by the Barrier Test Consortium Limited. The
results of this research have been published in a peer-reviewed
60 s, beginning no sooner than 1 min from the start of the
filtrate aerosol measurement. The challenge aerosol concentra- journal. This research demonstrated that testing the barrier
performance of porous packaging materials using microorgan-
tion is reported as the average concentration of the challenge
aerosol over a time period of not less than 45 s, beginning no isms correlates with measuring the filtration efficiency of the
materials.
sooner than 1 min from the start of the challenge measurement.
Challenge concentrations measured immediately before and 6.2 This test method does not require the use of microbio-
logical method; in addition, the test method can be conducted
after each filtrate concentration measurement are averaged to
determine the challenge concentration for a given flow rate. in a rapid and timely manner.
5.2.2 When using two particle counters, the challenge and
filtrate aerosols are counted continuously by dedicated particle
“Definition of a Correlation Between Microbiological and Physical Particulate
counters. The challenge and filtrate aerosol concentrations are
Barrier Performances for Porous Medical Packaging Materials,” PDA J Pharm Sci
reported as the average concentration of the challenge or Technol, Vol 56, No. 1, 2002, Jan-Feb, 11-9.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
F2638 – 07
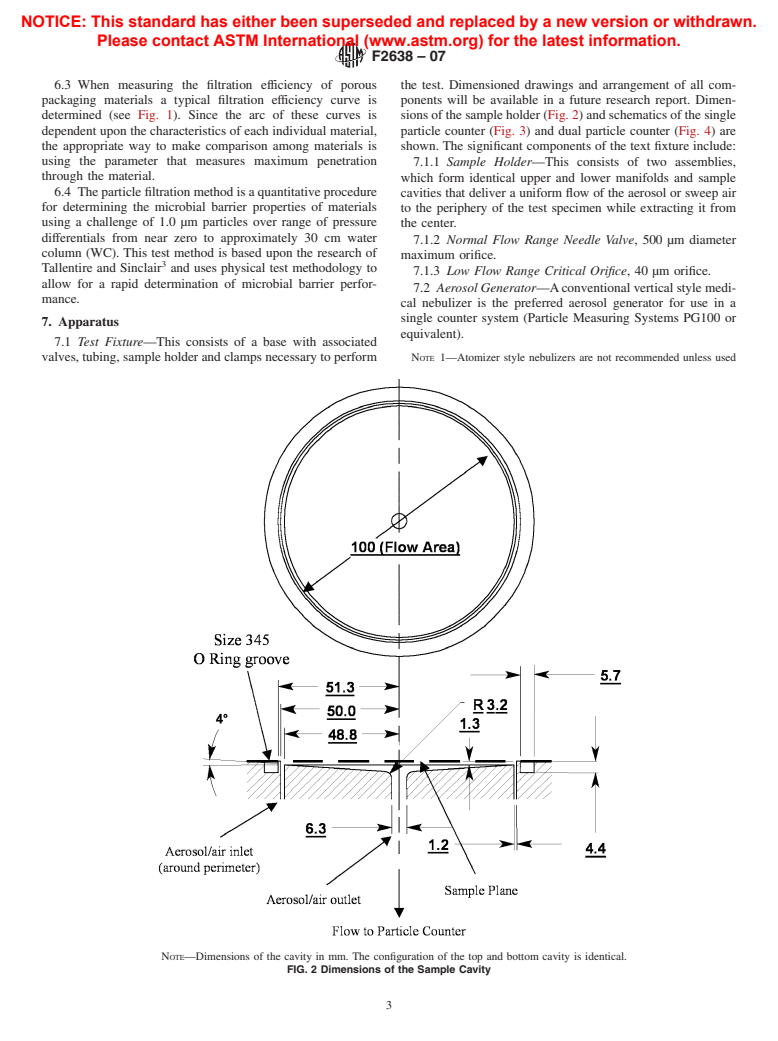
6.3 When measuring the filtration efficiency of porous the test. Dimensioned drawings and arrangement of all com-
packaging materials a typical filtration efficiency curve is ponents will be available in a future research report. Dimen-
determined (see Fig. 1). Since the arc of these curves is sions of the sample holder (Fig. 2) and schematics of the single
dependent upon the characteristics of each individual material, particle counter (Fig. 3) and dual particle counter (Fig. 4) are
the appropriate way to make comparison among materials is shown. The significant components of the text fixture include:
using the parameter that measures maximum penetration
7.1.1 Sample Holder—This consists of two assemblies,
through the material.
which form identical upper and lower manifolds and sample
6.4 Theparticlefiltrationmethodisaquantitativeprocedure cavities that deliver a uniform flow of the aerosol or sweep air
for determining the microbial barrier properties of materials
to the periphery of the test specimen while extracting it from
using a challenge of 1.0 µm particles over range of pressure the center.
differentials from near zero to approximately 30 cm water
7.1.2 Normal Flow Range Needle Valve, 500 µm diameter
column (WC). This test method is based upon the research of
maximum orifice.
Tallentire and Sinclair and uses physical test methodology to
7.1.3 Low Flow Range Critical Orifice, 40 µm orifice.
allow for a rapid determination of microbial barrier perfor-
7.2 Aerosol Generator—Aconventional vertical style medi-
mance.
cal nebulizer is the preferred aerosol generator for use in a
single counter system (Particle Measuring Systems PG100 or
7. Apparatus
equivalent).
7.1 Test Fixture—This consists of a base with associated
valves, tubing, sample holder and clamps necessary to perform NOTE 1—Atomizer style nebulizers are not recommended unless used
NOTE—Dimensions of the cavity in mm. The configuration of the top and bottom cavity is identical.
FIG. 2 Dimensions of the Sample Cavity
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
F2638 – 07
FIG. 3 Equipment Configuration for a Single Particle Counter—Method A
with a dual particle counter system as they exhibit sudden, unpredictable
7.4 Data Logging—The elapsed test time, the pressure
changes in aerosol concentration.
differential, the total challenge particles, and/or the total filtrate
7.3 Particle Counter—The particle counter required for this particles shall be recorded every 6 s. When using the Lasair
test method must be capable of distinguishing between the particle counters, the 1.0 µm PSL particles are counted in both
residue from water droplets and the polystyrene latex (PSL)
the 0.7 to 1.0 µm and the 1.0 to 2.0 µm size ranges. Therefore,
particles (Particle Measuring Systems Lasair series of counters
both counts shall be recorded and totaled.
orequivalent).Theparticlecountershouldhaveaflowdemand
7.5 Manometer—A precision manometer with a minimum
that approximates the flow through the test specimen at
range of 0 to 5 cm (0 to 2 in.)WC and an accuracy of 0.005 cm
maximum penetration. If the particle counter sorts particles by
(0.002 in.) WC to monitor the pressure difference across the
size, it must be determined in which size ranges the PSL
sample.
particles reside.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
F2638 – 07
FIG. 4 Equipment Configuration for Dual Particle Counters—Method B
7.6 Pressure Regulator—Precision regulator capable of de- 8.3 Concentrate suspension of 1 µm PSL particles (Duke
Scientific 3K1000, 5100A, and G0100 have all been found
livering 1.0 standard litre per minute at pressures up to 3 bar.
satisfactory).
7.7 ULPA Filter—Required to remove ambient particles.
8.4 Distilled water sufficiently free of dissolved material.
7.8 Buna N or Nitrile Rubber SAE Standard AS 568A
8.5 Porous packaging material.
Size–345 O-rings—Provide a seal between the challenge and
filtrate sides of the test.
9. Apparatus Preparation
9.1 Apparatus should be assembled as seen in Fig. 3 (single
8. Materials
particle counter) or Fig. 4 (dual particle counter).
8.1 Particle free, dry compressed air.
9.2 Material Preparation:
8.2 Tween 20 or sodium dodecylsulfate (SDS). 9.2.1 Surfactant Solution:
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Please contact ASTM International (www.astm.org) for the latest information.
F2638 – 07
9.2.1.1 Prepare a 0.02 % v/v solution of surfactant (Tween fore, an estimate must be made of the challenge aerosol
20, SDS, or equivalent) in distilled water daily. concentration at the time of the filtrate measurement.
9.2.1.2 Aerosolize the surfactant solution and determine the
11.1.2 Setupequipmentfor1particlecountermode,use0.7
particle size distribution of this solution by measuring the
to 1.0 µm and 1.0 to 2.0 µm bin data, record Lasair and
challenge aerosol. Ideally there should be no particles over 0.7
manometer data every 6 s. Record pressure drop across sample
µm in diameter detected. The aim is no more than 2 such
during each 6-s sample length while counting particles in
particles detected within any 6-s period. Monitor surfactant
filtrate stream.
solution for 1 min.
11.1.3 Testdistilledwater/surfactanttoensurewaterisclean
9.2.1.3 Table 1 is an example of the size distribution of
as described in 9.2.1.
surfactant solution suitable for use, each row being a 6-s
11.1.4 Prepare appropriate concentration (200 to 8000
counting interval.
particles/mL) of PSL suspension and confirm that the particle
9.2.2 Particle Suspension:
9.2.2.1 Prepare a suspension of 1 µm PSL particles in the counts are within 3 % as described in 9.2.2.3.
surfactant solution described above.
11.1.5 Open sample holder and place sample in the sample
holder.
NOTE 2—This solution is to be made fresh daily. When making the
suspension from a highly concentrated source (such as Duke Scientific
11.1.6 Select High Flow Range.
5100A) some of the particles will have agglomerated into aggregates
11.1.7 Start aerosol flow, set Particle Counter to count
consisting of multiple particles. To ensure the aerosol consists of particles
Challenge.
havingonlyonePSLparticle,placethebottlecontainingthesolutioninan
ultrasonic bath for 15 s. This will disassociate the particles.
11.1.8 Close the venturi needle valve and increase inlet air
pressure to 3 bar, open the needle valve until pressur
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.