ASTM F2638-12
(Test Method)Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier
SIGNIFICANCE AND USE
This test method has been developed as a result of research performed by Air Dispersion Limited (Manchester, UK) and funded by the Barrier Test Consortium Limited. The results of this research have been published in a peer-reviewed journal. This research demonstrated that testing the barrier performance of porous packaging materials using microorganisms correlates with measuring the filtration efficiency of the materials.
This test method does not require the use of microbiological method; in addition, the test method can be conducted in a rapid and timely manner.
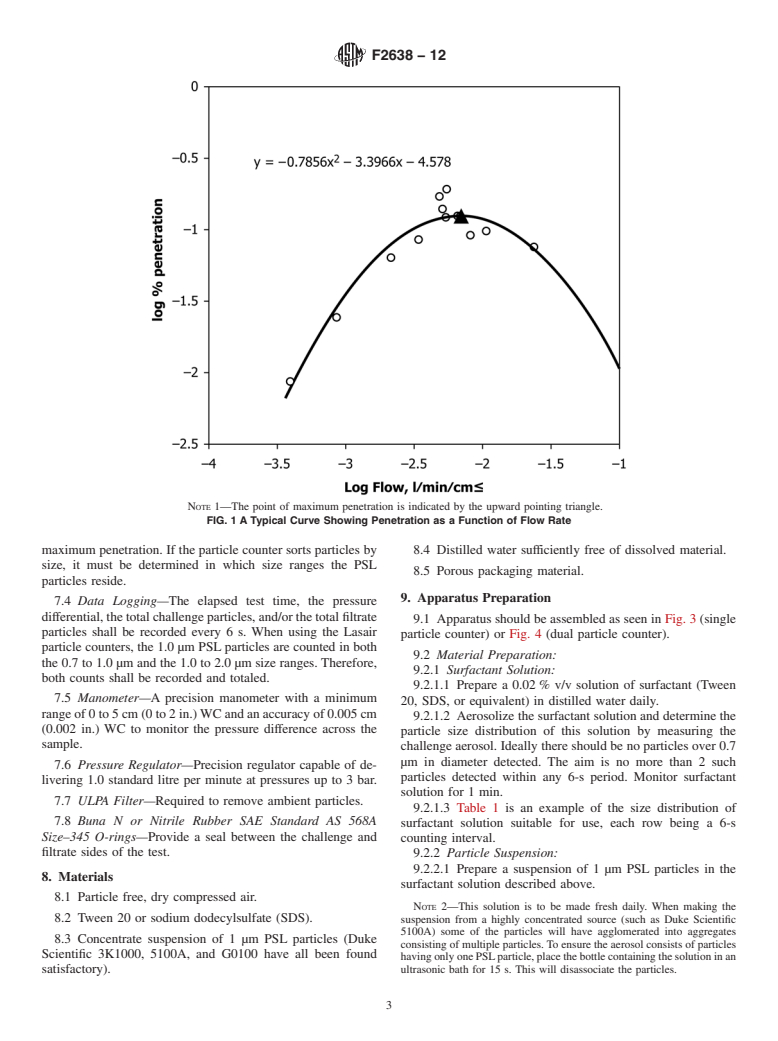
When measuring the filtration efficiency of porous packaging materials a typical filtration efficiency curve is determined (see Fig. 1). Since the arc of these curves is dependent upon the characteristics of each individual material, the appropriate way to make comparison among materials is using the parameter that measures maximum penetration through the material.
The particle filtration method is a quantitative procedure for determining the microbial barrier properties of materials using a challenge of 1.0 µm particles over range of pressure differentials from near zero to approximately 30 cm water column (WC). This test method is based upon the research of Tallentire and Sinclair and uses physical test methodology to allow for a rapid determination of microbial barrier performance.
SCOPE
1.1 This test method measures the aerosol filtration performance of porous packaging materials by creating a defined aerosol of 1.0 μm particles and assessing the filtration efficiency of the material using either single or dual particle counters.
1.2 This test method is applicable to porous materials used to package terminally sterilized medical devices.
1.3 The intent of this test method is to determine the flow rate through a material at which maximum penetration occurs. The porous nature of some materials used in sterile packaging applications might preclude evaluation by means of this test method. The maximum penetration point of a particular material could occur at a flow rate that exceeds the flow capacity of the test apparatus. As such, this test method may not be useful for evaluating the maximum penetration point of materials with a Bendtsen flow rate above 4000 mL/min as measured by ISO 5636–3.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2638 − 12
StandardTest Method for
Using Aerosol Filtration for Measuring the Performance of
Porous Packaging Materials as a Surrogate Microbial
1
Barrier
This standard is issued under the fixed designation F2638; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to
1.1 This test method measures the aerosol filtration perfor-
Determine the Precision of a Test Method
mance of porous packaging materials by creating a defined
3
2.2 ISO Standard:
aerosol of 1.0 µm particles and assessing the filtration effi-
ISO 5636–3 Paper and Board—Determination of Air Per-
ciency of the material using either single or dual particle
meance (Medium Range)—Part 3: Bendtsen Method
counters.
1.2 This test method is applicable to porous materials used
3. Terminology
to package terminally sterilized medical devices.
3.1 Definitions:
1.3 The intent of this test method is to determine the flow
3.1.1 challenge aerosol—a sufficient quantity of aerosolized
rate through a material at which maximum penetration occurs.
1.0 µm particles that enable effective particle counting in the
The porous nature of some materials used in sterile packaging
filtrate aerosol.
applications might preclude evaluation by means of this test
3.1.2 filtrate aerosol—particlesthatremainaerosolizedafter
method. The maximum penetration point of a particular mate-
passage through the test specimen.
rial could occur at a flow rate that exceeds the flow capacity of
the test apparatus.As such, this test method may not be useful 3.1.3 maximum penetration—the highest percent concentra-
forevaluatingthemaximumpenetrationpointofmaterialswith tionofparticlesinthefiltrateaerosolwhenaspecimenistested
a Bendtsen flow rate above 4000 mL/min as measured by over a range of pressure differentials or air flow rates.
ISO 5636–3.
3.2 Abbreviations and Symbols:
1.4 The values stated in SI units are to be regarded as the
Symbol Unit Description
C n Average particle count of the challenge aerosol
standard. The values given in parentheses are for information
S
when using a single particle counter (Method A).
only.
C n Average particle count of the filtrate aerosol.
F
C n Average particle count of the challenge aerosol.
1.5 This standard does not purport to address all of the C
C N Average particle count of the filtrate aerosol prior to
LR
safety concerns, if any, associated with its use. It is the
correction for dilution.
responsibility of the user of this standard to establish appro-
R % Percentage of particles from the challenge aerosol
that remain in the filtrate aerosol.
priate safety and health practices and determine the applica-
R % The calculated maximum of R.
M
bility of regulatory limitations prior to use.
P cm WC Pressure differential across a test specimen due to
1
the air flow required by the particle counter.
2. Referenced Documents P cm WC Pressure differential across a test specimen.
2
F L/m/cm Air flow rate through the test specimen.
2
2.1 ASTM Standards: 2
F L/m/cm Air flow rate required by the particle counter when
1
E177 Practice for Use of the Terms Precision and Bias in measuring the filtrate aerosol.
2
F L/m/cm Air flow rate at which maximum penetration occurs.
M
1 4. Safety
This test method is under the jurisdiction ofASTM Committee F02 on Flexible
Barrier Packaging and is the direct responsibility of Subcommittee F02.15 on
4.1 The waste and the vacuum venturi vents for the test
Chemical/Safety Properties.
equipment described in this test method emit an aerosol of
Current edition approved May 1, 2012. Published June 2012. Originally
approved in 2007. Last previous edition approved in 2007 as F2638 – 07. DOI: polystyrene particles and salt residues. These aerosols should
10.1520/F2638-12.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2638 − 12
4
be exhausted from any enclosed environment or collected and journal. This research demonstrated that testing the barrier
filtered to remove all particles. performance of porous packaging materials using microorgan-
isms correlates with measuring th
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:F2638–07 Designation: F2638 – 12
Standard Test Method for
Using Aerosol Filtration for Measuring the Performance of
Porous Packaging Materials as a Surrogate Microbial
1
Barrier
This standard is issued under the fixed designation F2638; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method measures the aerosol filtration performance of porous packaging materials by creating a defined aerosol
of 1.0 µm particles and assessing the filtration efficiency of the material using either single or dual particle counters.
1.2 This test method is applicable to porous materials used to package terminally sterilized medical devices.
1.3 The intent of this test method is to determine the flow rate through a material at which maximum penetration occurs. The
porous nature of some materials used in sterile packaging applications might preclude evaluation by means of this test method.
The maximum penetration point of a particular material could occur at a flow rate that exceeds the flow capacity of the test
apparatus.As such, this test method may not be useful for evaluating the maximum penetration point of materials with a Bendtsen
flow rate above 4000 mL/min as measured by ISO 5636–3.
1.4 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ISO Standard:ASTM Standards:
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3
2.2 ISO Standard:
ISO 5636–3 Paper and Board—Determination of Air Permeance (Medium Range)—Part 3: Bendtsen Method
3. Terminology
3.1 Definitions:
3.1.1 challengeaerosol—asufficientquantityofaerosolized1.0µmparticlesthatenableeffectiveparticlecountinginthefiltrate
aerosol.
3.1.2 filtrate aerosol—particles that remain aerosolized after passage through the test specimen.
3.1.3 maximum penetration—thehighestpercentconcentrationofparticlesinthefiltrateaerosolwhenaspecimenistestedover
a range of pressure differentials or air flow rates.
3.2 Abbreviations and Symbols:
Symbol Unit Description
C n Average particle count of the challenge aerosol
S
when using a single particle counter (Method A).
C n Average particle count of the filtrate aerosol.
F
C n Average particle count of the challenge aerosol.
C
1
This test method is under the jurisdiction of ASTM Committee F02 on Flexible Barrier Packaging and is the direct responsibility of Subcommittee F02.15 on
Chemical/Safety Properties.
Current edition approved Aug. 1, 2007. Published September 2007. DOI: 10.1520/F2638-07.
CurrenteditionapprovedMay1,2012.PublishedJune2012.Originallyapprovedin2007.Lastpreviouseditionapprovedin2007asF 2638 – 07.DOI:10.1520/F2638-12.
2
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
2
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
“DefinitionofaCorrelationBetweenMicrobiologicalandPhysicalParticulateBarrierPerformancesforPorousMedicalPackagingMaterials,”PDAJPharmSciTechnol,
Vol 56, No. 1, 2002, Jan-Feb, 11-9.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F2638 – 12
Symbol Unit Description
C N Average particle count of the filtrate aerosol prior to
LR
correction for dilution.
R % Percentage of particles from the challenge aerosol
that remain in the filtrate aerosol.
R % The calculated maximum of R.
M
P cm WC Pressure differential across a test specimen due to
1
the air flow required by the particle counter.
P cm W
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.