ASTM F623-99(2013)
(Specification)Standard Performance Specification for Foley Catheter
Standard Performance Specification for Foley Catheter
SCOPE
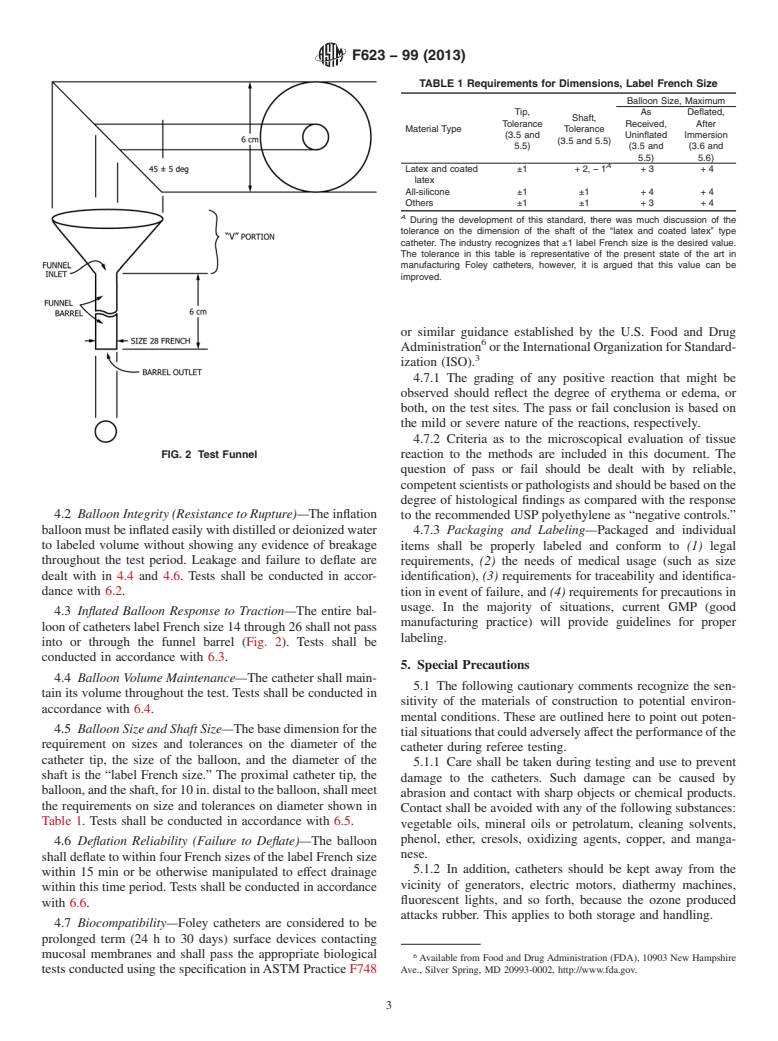
1.1 This performance specification establishes performance requirements for the short-term utilization of a single-use, balloon-retention catheter, French sizes 12 through 26 inclusive, used by the medical professions for providing a means of bladder drainage by means of the urethra. The product is manufactured in various sizes and materials such as latex, silicone, rubber, and various polymers (as well as combinations of these) and is provided nonsterile for sterilization and sterile for single use only. Catheters whose surface has been chemically treated to effect biocompatibility or microbial properties may be tested to this specification.
1.2 Exclusions—Long-term indwelling usage (over 30 days) is encountered with this product, but not commonly, and is therefore considered an exception to this specification. Similarly, the use of such catheters for nonurethral catheterization (such as for nephrostomy, suprapubic cystostomy, ureterostomy, gastrostomy, enemas, and so forth) is excluded from the scope of this specification. Likewise, three lumen catheters, 30-cm3 balloon and pediatric catheters, and catheters whose surface has been chemically treated to enhance their lubricity have not been tested to this specification and excluded from the scope of this specification and will require separate standard development.
1.3 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F623 −99 (Reapproved 2013)
Standard Performance Specification for
1
Foley Catheter
ThisstandardisissuedunderthefixeddesignationF623;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The objective of this specification is to describe those product requirements and associated test
3
methods that will ensure the safety and effectiveness of a disposable, 5-cm (mL) balloon,
retention-type catheter used in urinary bladder drainage.
This specification includes referee test methods that can be used to determine compliance with the
stated performance requirements. Note that the test methods are not to be construed as production
methods, quality control techniques, or manufacturer’s lot release criteria. The product parameters
addressed by the standard include those determined by the FDA Panel on Review of
Gastroenterological-Urological Devices to be pertinent to the proposed classification of the Foley
catheter to FDA Class II standards, plus other parameters determined by the ASTM task force to be
pertinent to the product.
This specification represents the state of the art at this time and is a minimum performance
specification. It is recognized that the document must remain dynamic; suggestions for revision are
encouraged,andshouldbedirectedtoCommitteeF04StaffManager,ASTM,100BarrHarborDr.,PO
Box C700, West Conshohocken, PA 19428–2959.
1. Scope whose surface has been chemically treated to enhance their
lubricityhavenotbeentestedtothisspecificationandexcluded
1.1 This performance specification establishes performance
from the scope of this specification and will require separate
requirements for the short-term utilization of a single-use,
standard development.
balloon-retention catheter, French sizes 12 through 26
inclusive, used by the medical professions for providing a 1.3 This standard may involve hazardous materials,
operations, and equipment. This standard does not purport to
means of bladder drainage by means of the urethra. The
product is manufactured in various sizes and materials such as address all of the safety concerns associated with its use. It is
the responsibility of the user of this standard to establish
latex, silicone, rubber, and various polymers (as well as
combinations of these) and is provided nonsterile for steriliza- appropriate safety and health practices and determine the
applicability of regulatory limitations prior to use.
tionandsterileforsingleuseonly.Catheterswhosesurfacehas
been chemically treated to effect biocompatibility or microbial
properties may be tested to this specification. 2. Referenced Documents
2
1.2 Exclusions—Long-termindwellingusage(over30days)
2.1 ASTM Standards:
is encountered with this product, but not commonly, and is F748PracticeforSelectingGenericBiologicalTestMethods
therefore considered an exception to this specification.
for Materials and Devices
Similarly, the use of such catheters for nonurethral catheter-
2.2 Other Documents:
ization (such as for nephrostomy, suprapubic cystostomy,
ISO/AAMI/ANSI 10993–1Biological Testing of Medical
ureterostomy, gastrostomy, enemas, and so forth) is excluded
and Dental Material and Devices — Part 1: Guidance on
from the scope of this specification. Likewise, three lumen 3
Selection of Tests
3
catheters,30-cm balloonandpediatriccatheters,andcatheters
1 2
This performance specification is under the jurisdiction of ASTM Committee For referenced ASTM standards, visit the ASTM website, www.astm.org, or
F04 on Medical and Surgical Materials and Devices and is the direct responsibility contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of Subcommittee F04.34 on Urological Materials and Devices. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Oct. 1, 2013. Published October 2013. Originally the ASTM website.
3
approved in 1981. Last previous edition approved in 2006 as F623–99 (2006). Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
DOI: 10.1520/F0623-99R13. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F623−99 (2013)
FIG. 1 Balloon Retention (Foley) Type Catheter
4
U.S. Pharmacopeia
26 0.341 (8.7)
3.1.4 lumen—the channel within a tube.
3. Terminology
3.1.5 proximal—refers to the balloon end of the catheter,
3.1 Definitions:
since when in position for clinical use, the balloon end is
3.1.1 balloon (Foley) catheter—an indwelling catheter re-
proximal or closest
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.