ASTM E2709-23
(Practice)Standard Practice for Demonstrating Capability to Comply with an Acceptance Procedure
Standard Practice for Demonstrating Capability to Comply with an Acceptance Procedure
ABSTRACT
This practice provides a general methodology for evaluating single-stage or multiple-stage acceptance procedures which involve a quality characteristic measured on a numerical scale. This methodology computes, at a prescribed confidence level, a lower bound on the probability of passing an acceptance procedure, using estimates of the parameters of the distribution of test results from a sampled population.
SIGNIFICANCE AND USE
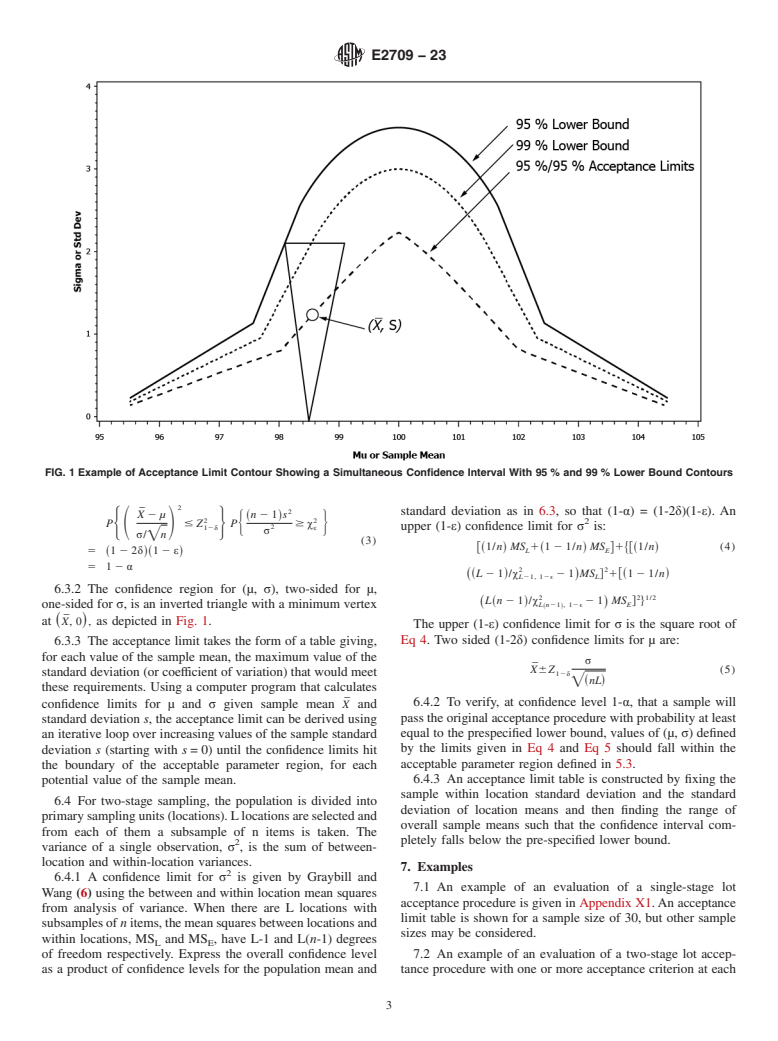
4.1 This practice considers inspection procedures that may involve multiple-stage sampling, where at each stage one can decide to accept or to continue sampling, and the decision to reject is deferred until the last stage.
4.1.1 At each stage there are one or more acceptance criteria on the test results; for example, limits on each individual test result, or limits on statistics based on the sample of test results, such as the average, standard deviation, or coefficient of variation (relative standard deviation).
4.2 The methodology in this practice defines an acceptance region for a set of test results from the sampled population such that, at a prescribed confidence level, the probability that a sample from the population will pass the acceptance procedure is greater than or equal to a prespecified lower bound.
4.2.1 Having test results fall in the acceptance region is not equivalent to passing the acceptance procedure, but provides assurance that a sample would pass the acceptance procedure with a specified probability.
4.2.2 This information can be used for process demonstration, validation of test methods, and qualification of instruments, processes, and materials.
4.2.3 This information can be used for lot release (acceptance), but the lower bound may be conservative in some cases.
4.2.4 If the results are to be applied to future test results from the same process, then it is assumed that the process is stable and predictable. If this is not the case then there can be no guarantee that the probability estimates would be valid predictions of future process performance.
4.3 This methodology was originally developed (1-4)3 for use in two specific quality characteristics of drug products in the pharmaceutical industry but will be applicable for acceptance procedures in all industries.
4.4 Mathematical derivations would be required that are specific to the individual criteria of each test.
SCOPE
1.1 This practice provides a general methodology for evaluating single-stage or multiple-stage acceptance procedures which involve a quality characteristic measured on a numerical scale. This methodology computes, at a prescribed confidence level, a lower bound on the probability of passing an acceptance procedure, using estimates of the parameters of the distribution of test results from a sampled population.
1.2 For a prescribed lower probability bound, the methodology can also generate an acceptance limit table, which defines a set of test method outcomes (for example, sample averages and standard deviations) that would pass the acceptance procedure at a prescribed confidence level.
1.3 This approach may be used for demonstrating compliance with in-process, validation, or lot-release specifications.
1.4 The system of units for this practice is not specified.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2709 − 23 An American National Standard
Standard Practice for
Demonstrating Capability to Comply with an Acceptance
1
Procedure
This standard is issued under the fixed designation E2709; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E2282 Guide for Defining the Test Result of a Test Method
1.1 This practice provides a general methodology for evalu-
3. Terminology
ating single-stage or multiple-stage acceptance procedures
3.1 Definitions—Unless otherwise noted in this standard, all
which involve a quality characteristic measured on a numerical
terms relating to quality and statistics are defined in Terminol-
scale. This methodology computes, at a prescribed confidence
ogy E456.
level, a lower bound on the probability of passing an accep-
3.1.1 characteristic, n—a property of items in a sample or
tance procedure, using estimates of the parameters of the
population which, when measured, counted or otherwise
distribution of test results from a sampled population.
observed, helps to distinguish between the items. E2282
1.2 For a prescribed lower probability bound, the method-
3.1.2 multiple-stage acceptance procedure, n—a procedure
ology can also generate an acceptance limit table, which
that involves more than one stage of sampling and testing a
defines a set of test method outcomes (for example, sample
given quality characteristic and one or more acceptance criteria
averages and standard deviations) that would pass the accep-
per stage.
tance procedure at a prescribed confidence level.
3.1.3 test method, n—a definitive procedure that produces a
1.3 This approach may be used for demonstrating compli-
test result. E2282
ance with in-process, validation, or lot-release specifications.
3.2 Definitions of Terms Specific to This Standard:
1.4 The system of units for this practice is not specified.
3.2.1 acceptable parameter region, n—the set of values of
1.5 This standard does not purport to address all of the
parameters characterizing the distribution of test results for
safety concerns, if any, associated with its use. It is the
which the probability of passing the acceptance procedure is
responsibility of the user of this standard to establish appro-
greater than a prescribed lower bound.
priate safety, health, and environmental practices and deter-
3.2.2 acceptance region, n—the set of values of parameter
mine the applicability of regulatory limitations prior to use.
estimates that will attain a prescribed lower bound on the
1.6 This international standard was developed in accor-
probability of passing an acceptance procedure at a prescribed
dance with internationally recognized principles on standard-
level of confidence.
ization established in the Decision on Principles for the
3.2.3 acceptance limit, n—the boundary of the acceptance
Development of International Standards, Guides and Recom-
region, for example, the maximum sample standard deviation
mendations issued by the World Trade Organization Technical
test results for a given sample mean.
Barriers to Trade (TBT) Committee.
4. Significance and Use
2. Referenced Documents
2
4.1 This practice considers inspection procedures that may
2.1 ASTM Standards:
involve multiple-stage sampling, where at each stage one can
E456 Terminology Relating to Quality and Statistics
decide to accept or to continue sampling, and the decision to
reject is deferred until the last stage.
4.1.1 At each stage there are one or more acceptance criteria
1
This practice is under the jurisdiction of ASTM Committee E11 on Quality and
on the test results; for example, limits on each individual test
Statistics and is the direct responsibility of Subcommittee E11.20 on Test Method
Evaluation and Quality Control.
result, or limits on statistics based on the sample of test results,
Current edition approved Nov. 1, 2023. Published November 2023. Originally
such as the average, standard deviation, or coefficient of
approved in 2009. Last previous edition approved in 2023 as E2709 – 19 (2023).
variation (relative standard deviation).
DOI: 10.1520/E2709-23.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.2 The methodology in this practice defines an acceptance
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
region for a set of test results from the sampled population such
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. th
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2709 − 19 (Reapproved 2023) E2709 − 23 An American National Standard
Standard Practice for
Demonstrating Capability to Comply with an Acceptance
1
Procedure
This standard is issued under the fixed designation E2709; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice provides a general methodology for evaluating single-stage or multiple-stage acceptance procedures which
involve a quality characteristic measured on a numerical scale. This methodology computes, at a prescribed confidence level, a
lower bound on the probability of passing an acceptance procedure, using estimates of the parameters of the distribution of test
results from a sampled population.
1.2 For a prescribed lower probability bound, the methodology can also generate an acceptance limit table, which defines a set
of test method outcomes (for example, sample averages and standard deviations) that would pass the acceptance procedure at a
prescribed confidence level.
1.3 This approach may be used for demonstrating compliance with in-process, validation, or lot-release specifications.
1.4 The system of units for this practice is not specified.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E456 Terminology Relating to Quality and Statistics
E2282 Guide for Defining the Test Result of a Test Method
E2586 Practice for Calculating and Using Basic Statistics
3. Terminology
3.1 Definitions—See Terminology—Unless otherwise E456 for a more extensive listing of terms in ASTM Committee E11
standards.noted in this standard, all terms relating to quality and statistics are defined in Terminology E456.
1
This practice is under the jurisdiction of ASTM Committee E11 on Quality and Statistics and is the direct responsibility of Subcommittee E11.20 on Test Method
Evaluation and Quality Control.
Current edition approved April 1, 2023Nov. 1, 2023. Published April 2023November 2023. Originally approved in 2009. Last previous edition approved in 20192023 as
E2709 – 19.E2709 – 19 (2023). DOI: 10.1520/E2709-19R23.10.1520/E2709-23.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2709 − 23
3.1.1 characteristic, n—a property of items in a sample or population which, when measured, counted or otherwise observed,
helps to distinguish between the items. E2282
¯
3.1.2 mean, n—of a population, μ, average or expected value of a characteristic in a population, of a sampleX, sum of the observed
values in a sample divided by the sample size. E2586
3.1.2 multiple-stage acceptance procedure, n—a procedure that involves more than one stage of sampling and testing a given
quality characteristic and one or more acceptance criteria per stage.
3.1.4 standard deviation, n—of a population, σ, the square root of the average or expected value of the squared deviation of a
variable from its mean – of a sample, s, the square root of the sum of the squared deviations of the observed values in the sample
divided by the sample size minus 1. E2586
3.1.3 test method, n—a definitive procedure that produces a test result. E2282
3.2 Definitions of Terms Specific to This Standard:
3.2.1 acceptable parameter region, n—the set of values of parameters characterizing the distribution of test results for which the
probability of passing the acceptance procedure is greater than a prescribed lower bound.
3.2.2 accepta
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.