ASTM D1394-76(2014)
(Test Method)Standard Test Methods for Chemical Analysis of White Titanium Pigments
Standard Test Methods for Chemical Analysis of White Titanium Pigments
ABSTRACT
These test methods cover procedures for the chemical analysis of white titanium dioxide pigments. The analytical procedures appear in the following order: sample preparation, qualitative analysis, moisture content determination, total titanium content determination by Jones Reductor and Aluminum Reduction methods, aluminum oxide content determination, and silica content determination. The reagents to be used shall include ammonium hydroxide, ammonium sulfate, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, sulfuric acid, tartaric acid, tin, zinc, carbon steel, iron, ferric sulfate solution, nitric acid, sodium oxalate, potassium permanganate, aluminum metal foil, ammonium thiocyanate indicator solution, ferritic ammonium sulfate solution, sodium bicarbonate solution, sulfuric acid, titanium dioxide, acetic acid, ammonium phosphate, EDTA solution, methyl orange indicator solution, sodium bisulfate monohydrate, sodium fluoride, xylenol orange indicator solution, and zinc sulfate solution.
SCOPE
1.1 These test methods cover procedures for the chemical analysis of white titanium dioxide pigments.
1.2 The analytical procedures appear in the following order:
Sections
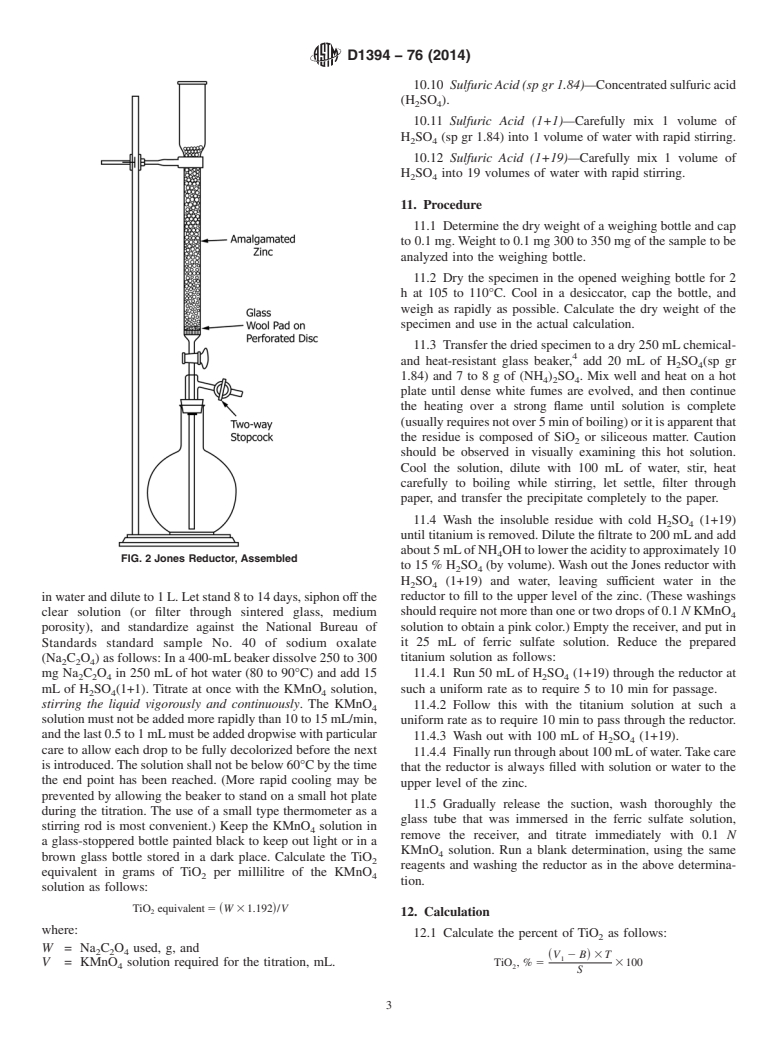
Preparation of Sample
4
Qualitative Analysis
5 and 6
Moisture
7
Total Titanium:
Jones Reductor Method
8 – 12
Aluminum Reduction Method
13 – 17
Aluminum Oxide
18 – 22
Silica
23 – 29
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. A specific hazard statement is given in Section 19.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D1394 − 76 (Reapproved 2014)
Standard Test Methods for
Chemical Analysis of White Titanium Pigments
This standard is issued under the fixed designation D1394; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope all reagents shall conform to the specifications of the Commit-
tee onAnalytical Reagents of theAmerican Chemical Society,
1.1 These test methods cover procedures for the chemical
where such specifications are available. Other grades may be
analysis of white titanium dioxide pigments.
used, provided it is first ascertained that the reagent is of
1.2 Theanalyticalproceduresappearinthefollowingorder:
sufficiently high purity to permit its use without lessening the
Sections
accuracy of the determination.
Preparation of Sample 4
Qualitative Analysis 5 and 6
3.2 Unless otherwise indicated, references to water shall be
Moisture 7
understood to mean reagent water conforming to Type IV of
Total Titanium:
Specification D1193.
Jones Reductor Method 8–12
Aluminum Reduction Method 13–17
Aluminum Oxide 18–22
4. Preparation of Sample
Silica 23–29
4.1 The sample shall, in all cases, be thoroughly mixed and
1.3 The values stated in SI units are to be regarded as
comminuted before taking portions for analysis.
standard. No other units of measurement are included in this
standard.
QUALITATIVE ANALYSIS
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
5. Reagents
responsibility of the user of this standard to establish appro-
5.1 Ammonium Hydroxide (sp gr 0.90)—Concentrated am-
priate safety and health practices and determine the applica-
monium hydroxide (NH OH).
bility of regulatory limitations prior to use. A specific hazard
statement is given in Section 19.
5.2 Ammonium Sulfate—((NH ) SO ).
4 2 4
2. Referenced Documents
5.3 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
chloric acid (HCl).
2.1 ASTM Standards:
D280Test Methods for Hygroscopic Moisture (and Other
5.4 Hydrogen Peroxide (30 %)—Concentrated hydrogen
Matter Volatile Under the Test Conditions) in Pigments
peroxide (H O ).
2 2
D1193Specification for Reagent Water
5.5 Hydrogen Sulfide (H S).
E50Practices for Apparatus, Reagents, and Safety Consid- 2
erations for Chemical Analysis of Metals, Ores, and
5.6 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid
Related Materials
(H SO ).
2 4
3. Reagents
5.7 Sulfuric Acid (1+19)—Carefully mix 1 volume of
H SO (sp gr 1.84) with 19 volumes of water.
3.1 Purity of Reagent—Reagent grade chemicals shall be 2 4
used in all tests. Unless otherwise indicated, it is intended that
5.8 Tartaric Acid.
5.9 Tin or Zinc Metal.
These test methods are under the jurisdiction of ASTM Committee D01 on
Paint and Related Coatings, Materials, and Applications and are the direct
responsibility of Subcommittee D01.31 on Pigment Specifications.
Current edition approved Dec. 1, 2014. Published December 2014. Originally
approved in 1956. Last previous edition approved in 2009 as D1394–76(2009). Reagent Chemicals, American Chemical Society Specifications, American
DOI: 10.1520/D1394-76R14. Chemical Society, Washington, DC. For suggestions on the testing of reagents not
For referenced ASTM standards, visit the ASTM website, www.astm.org, or listed by the American Chemical Society, see Analar Standards for Laboratory
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
Standards volume information, refer to the standard’s Document Summary page on and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
the ASTM website. MD.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D1394 − 76 (2014)
6. Procedure
6.1 Place about 0.5 g of the sample in a 250-mL glass
beaker, and add 20 mLof H SO (sp gr 1.84) and 7 to8gof
2 4
(NH ) SO . Mix well and boil for a few minutes. The sample
4 2 4
should go completely into solution; a residue denotes the
presenceofsilicondioxide(SiO )orsiliceousmatter.Coolthe
solution,dilutewith100mLofwater,heattoboiling,letsettle,
filter, wash with hot H SO (1+19) until free of titanium, and
2 4
test the residue for lead, etc.
6.2 Test the filtrate for calcium, zinc, iron, chromium, etc.,
by the regular methods of qualitative analysis. For the iron
determinationaddtoaportionofthefiltrate5goftartaricacid,
render slightly ammoniacal, pass in H S in excess, and digest
on a steam bath. No precipitate after 30 min indicates the
absence of iron, nickel, cobalt, lead, copper, etc. A black
precipitate readily soluble in dilute HCl denotes iron. For
titanium, test a small portion of the original filtrate with H O
2 2
(aclearyellow-orangecolorshouldresult)andanotherportion
withmetallictinorzinc(apalebluetovioletcolorationshould
result). Negative results should be shown for sulfide,
carbonate, or appreciable water-soluble matter.
MOISTURE
7. Procedure
7.1 Determine moisture and other volatile matter in accor-
dance with Test Method A of Test Methods D280.
TOTAL TITANIUM BY THE JONES REDUCTOR
FIG. 1 Jones Reduction
METHOD
8. Scope
10. Reagents
8.1 This method gives results similar to those obtained with
10.1 Ammonium Hydroxide (sp gr 0.90)—Concentratedam-
the Aluminum Reduction Method, Sections13–17.
monium hydroxide (NH OH).
9. Apparatus
10.2 Ammonium Sulfate ((NH ) SO ).
4 2 4
9.1 Jones Reductor having a zinc column at least 450 mm
10.3 Carbon Steel or Iron—Pure iron or plain carbon steel.
in length, and 19 mm in diameter (Fig. 1 and Fig. 2). The
10.4 Ferric Sulfate Solution (1 mL = 0.02 g Fe)—Dissolve
filtering pad must be tight enough to hold all the particles of
20 g of iron or carbon steel in a slight excess of HCl, oxidize
amalgamated zinc resting on it, and may be made of asbestos
with approximately 12 mL of HNO , add about 80 mL of
or, preferably, glass-wool supported by platinum gauze or a
H SO , and heat to dense white fumes. Cool, dilute with water
2 4
perforated porcelain plate. Use the least amount (0.1 to 1.0%)
to 1 L, digest on a steam bath until sulfates are dissolved, and
of mercury that will enable satisfactory control of hydrogen
filter if necessary. To oxidize any ferrous iron that may be
evolution, since heavy amalgamation tends to reduce the rate
present, add 0.1 N KMnO solution until a faint pink color
ofreaction.Preparetheamalgambywashing20-meshzincfor
persists for 5 min. Ferric ammonium sulfate (FeNH (SO ) ·
4 4 2
1mininenough1 NHCltocoverit,addingtheproperamount
12H O) may also be used to prepare this solution (See 15.4).
of 0.25 M mercuric nitrate or chloride solution, and stirring
rapidly for 3 min. Decant the solution and wash the amalgam
10.5 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
with water and store under water to which a few drops of HCl chloric acid (HCl).
have been added. After using, keep the reductor filled with
10.6 Iron or Carbon Steel—Pure iron or plain carbon steel.
water when not in use, in order that basic salts will not be
10.7 Nitric Acid (sp gr 1.42)—Concentrated nitric acid
formed and clog it.
(HNO ).
10.8 Sodium Oxalate—National Institute of Standards and
Borosilicate glass has been found satisfactory for this purpose.
Technology standard reference material No. 40 of sodium
Treadwell,F.P.,andHall,WilliamT., Qualitative Analysis,JohnWiley&Sons,
Inc., New York, NY, Vol. 1, Ninth English Ed., 1937. oxalate (Na C O ).
2 2 4
Directions for preparing a Jones Reductor may be found in Hillebrand, W. F.,
10.9 Potassium Permanganate, Standard Reference Mate-
et al., Applied Inorganic Analysis, JohnWiley & Sons, Inc., NewYork, NY, Second
Ed., 1953, p. 108. rial(0.1 N,1mL=0.008gTiO )—Dissolve3.16gofKMnO
2 4
D1394 − 76 (2014)
10.10 Sulfuric Acid (sp gr 1.84)—Concentratedsulfuricacid
(H SO ).
2 4
10.11 Sulfuric Acid (1+1)—Carefully mix 1 volume of
H SO (sp gr 1.84) into 1 volume of water with rapid stirring.
2 4
10.12 Sulfuric Acid (1+19)—Carefully mix 1 volume of
H SO into 19 volumes of water with rapid stirring.
2 4
11. Procedure
11.1 Determine the dry weight of a weighing bottle and cap
to0.1mg.Weightto0.1mg300to350mgofthesampletobe
analyzed into the weighing bottle.
11.2 Dry the specimen in the opened weighing bottle for 2
h at 105 to 110°C. Cool in a desiccator, cap the bottle, and
weigh as rapidly as possible. Calculate the dry weight of the
specimen and use in the actual calculation.
11.3 Transferthedriedspecimentoadry250mLchemical-
and heat-resistant glass beaker, add 20 mL of H SO (sp gr
2 4
1.84) and 7 to8gof(NH ) SO . Mix well and heat on a hot
4 2 4
plate until dense white fumes are evolved, and then continue
the heating over a strong flame until solution is complete
(usuallyrequiresnotover5minofboiling)oritisapparentthat
the residue is composed of SiO or siliceous matter. Caution
should be observed in visually examining this hot solution.
Cool the solution, dilute with 100 mL of water, stir, heat
carefully to boiling while stirring, let settle, filter through
paper, and transfer the precipitate completely to the paper.
11.4 Wash the insoluble residue with cold H SO (1+19)
2 4
untiltitaniumisremoved.Dilutethefiltrateto200mLandadd
about5mLofNH OHtolowertheaciditytoapproximately10
FIG. 2 Jones Reductor, Assembled
to 15% H SO (by volume).Wash out the Jones reductor with
2 4
H SO (1+19) and water, leaving sufficient water in the
2 4
reductor to fill to the upper level of the zinc. (These washings
inwateranddiluteto1L.Letstand8to14days,siphonoffthe
clear solution (or filter through sintered glass, medium shouldrequirenotmorethanoneortwodropsof0.1 NKMnO
solution to obtain a pink color.) Empty the receiver, and put in
porosity), and standardize against the National Bureau of
it 25 mL of ferric sulfate solution. Reduce the prepared
Standards standard sample No. 40 of sodium oxalate
titanium solution as follows:
(Na C O )asfollows:Ina400-mLbeakerdissolve250to300
2 2 4
mg Na C O in 250 mLof hot water (80 to 90°C) and add 15 11.4.1 Run 50 mLof H SO (1+19) through the reductor at
2 2 4 2 4
mL of H SO (1+1). Titrate at once with the KMnO solution, such a uniform rate as to require 5 to 10 min for passage.
2 4 4
stirring the liquid vigorously and continuously. The KMnO
11.4.2 Follow this with the titanium solution at such a
solutionmustnotbeaddedmorerapidlythan10to15mL/min,
uniform rate as to require 10 min to pass through the reductor.
andthelast0.5to1mLmustbeaddeddropwisewithparticular
11.4.3 Wash out with 100 mL of H SO (1+19).
2 4
care to allow each drop to be fully decolorized before the next
11.4.4 Finallyrunthroughabout100mLofwater.Takecare
isintroduced.Thesolutionshallnotbebelow60°Cbythetime
that the reductor is always filled with solution or water to the
the end point has been reached. (More rapid cooling may be
upper level of the zinc.
prevented by allowing the beaker to stand on a small hot plate
11.5 Gradually release the suction, wash thoroughly the
during the titration. The use of a small type thermometer as a
glass tube that was immersed in the ferric sulfate solution,
stirring rod is most convenient.) Keep the KMnO solution in
remove the receiver, and titrate immediately with 0.1 N
a glass-stoppered bottle painted black to keep out light or in a
KMnO solution. Run a blank determination, using the same
brown glass bottle stored in a dark place. Calculate the TiO
reagents and washing the reductor as in the above determina-
equivalent in grams of TiO per millilitre of the KMnO
2 4
tion.
solution as follows:
TiO equivalent 5 ~W 31.192!/V
12. Calculation
where:
12.1 Calculate the percent of TiO as follows:
W =Na C O used, g, and
2 2 4
V 2 B 3 T
~ !
V = KMnO solution required for the titration, mL. TiO,% 5 3100
4 2
S
D1394 − 76 (2014)
where: 15.7 Sodium Bicarbonate Solution—Make up a saturated
solution at the time of analysis. About 10 g of sodium
V = KMnO solutionrequiredfortitrationofspecimen,mL
1 4
bicarbonate (NaHCO ) to 90 g of water is required.
B = KMnO solution required for titration of the blank, mL
T =TiO equivalent of the KMnO solution, g/mL, and
2 4
15.8 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid
S = dried specimen, g.
(H SO ).
2 4
12.2 The results calculated in accordance with 12.1 will
15.9 Titanium Dioxide (TiO )—National Bureau of Stan-
include iron, chromium, arsenic, and any other substance that
dards standard sample No. 154 of titanium dioxide.
isreducedbyzincandacid.However,appreciablequantitiesof
interferingmaterialsarenotlikelytobeencounteredinnormal,
16. Procedure
white titanium pigments.
16.1 Determine the dry weight of the weighing bottle and
TOTAL TITANIUM BY THE ALUMINUM
cap.Weigh to the nearest 0.1 mg, 190 to 210 mg of the sample
REDUCTION METHOD
to be analyzed into the weighing bottle.
13. Scope 16.2 Drythespecimenintheopenweighingbottlefor2hat
105to110°C.Coolinadesiccator,capthebottle,andweighas
13.1 This method gives results similar to those obtained
rapidly as possible. Calculate the dry weight of the specimen
with the Jones Reductor Method (Sections8–12).
and use in the actual calculation.
14. Apparatus
16.3 Transfer the dry specimen to a 500-mL dry, wide-
mouthErlenmeyerflask.Add7to9gof(NH ) SO and20mL
14.1 Delivery Tube, made of about 4-mm inside diameter 4 2 4
of H SO . Mix well, heat on a hot plate until dense white
glass tubing bent so that there is a horizontal run of about 6 in.
2 4
fumes are evolved, and continue the heating over a strong
(152mm)andaverticaldropofabout3in.(76mm)atoneend,
flame until solution is complete (usually requires not over 5
and a vertical drop of about 6 in. at the other end.
minofboiling)oritisapparentthattheresidueiscomposedof
14.2 Weighing Bottle, wide-mouth, with an external-fitting
SiO or siliceous matter. Cool and, with caution, add 120 mL
cap, and no larger than necessary for the required amount of
of water and 20 mL of HCl. Bring to a boil and remove from
sample.
heat.
15. Reagents
16.4 Insert the short end of the delivery tube into one hole
of a two-hole rubber stopper suitable for the Erlenmeyer flask.
15.1 Aluminum Metal Foil, electrolytic grade.
Insertaglassrodwithaslighthookorcollaratthebottomend
15.2 Ammonium Sulfate—((NH ) SO ).
4 2 4
intotheotherholeofthestopperinsuchawaythatthebottom
15.3 Ammonium Thiocyanate Indicat
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D1394 − 76 (Reapproved 2009) D1394 − 76 (Reapproved 2014)
Standard Test Methods for
Chemical Analysis of White Titanium Pigments
This standard is issued under the fixed designation D1394; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope
1.1 These test methods cover procedures for the chemical analysis of white titanium dioxide pigments.
1.2 The analytical procedures appear in the following order:
Sections
Preparation of Sample 4
Qualitative Analysis 5 and 6
Moisture 7
Total Titanium:
Jones Reductor Method 8 – 12
Aluminum Reduction Method 13 – 17
Aluminum Oxide 18 – 22
Silica 23 – 29
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. A specific hazard statement is given in Section 19.
2. Referenced Documents
2.1 ASTM Standards:
D280 Test Methods for Hygroscopic Moisture (and Other Matter Volatile Under the Test Conditions) in Pigments
D1193 Specification for Reagent Water
E50 Practices for Apparatus, Reagents, and Safety Considerations for Chemical Analysis of Metals, Ores, and Related Materials
3. Reagents
3.1 Purity of Reagent—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
3.2 Unless otherwise indicated, references to water shall be understood to mean reagent water conforming to Type IV of
Specification D1193.
4. Preparation of Sample
4.1 The sample shall, in all cases, be thoroughly mixed and comminuted before taking portions for analysis.
These test methods are under the jurisdiction of ASTM Committee D01 on Paint and Related Coatings, Materials, and Applications and are the direct responsibility of
Subcommittee D01.31 on Pigment Specifications.
Current edition approved June 1, 2009Dec. 1, 2014. Published June 2009December 2014. Originally approved in 1956. Last previous edition approved in 20032009 as
D1394 – 76 (2003).(2009). DOI: 10.1520/D1394-76R09.10.1520/D1394-76R14.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’sstandard’s Document Summary page on the ASTM website.
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For suggestions on the testing of reagents not listed by
the American Chemical Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D1394 − 76 (2014)
QUALITATIVE ANALYSIS
5. Reagents
5.1 Ammonium Hydroxide (sp gr 0.90)—Concentrated ammonium hydroxide (NH OH).
5.2 Ammonium Sulfate—((NH ) SO ).
4 2 4
5.3 Hydrochloric Acid (sp gr 1.19)—Concentrated hydrochloric acid (HCl).
5.4 Hydrogen Peroxide (30 %)—Concentrated hydrogen peroxide (H O ).
2 2
5.5 Hydrogen Sulfide (H S).
5.6 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid (H SO ).
2 4
5.7 Sulfuric Acid (1+19)—Carefully mix 1 volume of H SO (sp gr 1.84) with 19 volumes of water.
2 4
5.8 Tartaric Acid.
5.9 Tin or Zinc Metal.
6. Procedure
6.1 Place about 0.5 g of the sample in a 250-mL glass beaker, and add 20 mL of H SO (sp gr 1.84) and 7 to 8 g of (NH ) SO .
2 4 4 2 4
Mix well and boil for a few minutes. The sample should go completely into solution; a residue denotes the presence of silicon
dioxide (SiO ) or siliceous matter. Cool the solution, dilute with 100 mL of water, heat to boiling, let settle, filter, wash with hot
H SO (1+19) until free of titanium, and test the residue for lead, etc.
2 4
6.2 Test the filtrate for calcium, zinc, iron, chromium, etc., by the regular methods of qualitative analysis. For the iron
determination add to a portion of the filtrate 5 g of tartaric acid, render slightly ammoniacal, pass in H S in excess, and digest on
a steam bath. No precipitate after 30 min indicates the absence of iron, nickel, cobalt, lead, copper, etc. A black precipitate readily
soluble in dilute HCl denotes iron. For titanium, test a small portion of the original filtrate with H O (a clear yellow-orange color
2 2
should result) and another portion with metallic tin or zinc (a pale blue to violet coloration should result). Negative results should
be shown for sulfide, carbonate, or appreciable water-soluble matter.
MOISTURE
7. Procedure
7.1 Determine moisture and other volatile matter in accordance with Test Method A of Test Methods D280.
TOTAL TITANIUM BY THE JONES REDUCTOR METHOD
8. Scope
8.1 This method gives results similar to those obtained with the Aluminum Reduction Method, Sections 13 – 17.
9. Apparatus
9.1 Jones Reductor having a zinc column at least 450 mm in length, and 19 mm in diameter (Fig. 1 and Fig. 2). The filtering
pad must be tight enough to hold all the particles of amalgamated zinc resting on it, and may be made of asbestos or, preferably,
glass-wool supported by platinum gauze or a perforated porcelain plate. Use the least amount (0.1 to 1.0 %) of mercury that will
enable satisfactory control of hydrogen evolution, since heavy amalgamation tends to reduce the rate of reaction. Prepare the
amalgam by washing 20-mesh zinc for 1 min in enough 1 N HCl to cover it, adding the proper amount of 0.25 M mercuric nitrate
or chloride solution, and stirring rapidly for 3 min. Decant the solution and wash the amalgam with water and store under water
to which a few drops of HCl have been added. After using, keep the reductor filled with water when not in use, in order that basic
salts will not be formed and clog it.
10. Reagents
10.1 Ammonium Hydroxide (sp gr 0.90)—Concentrated ammonium hydroxide (NH OH).
10.2 Ammonium Sulfate ((NH ) SO ).
4 2 4
10.3 Carbon Steel or Iron—Pure iron or plain carbon steel.
Borosilicate glass has been found satisfactory for this purpose.
Treadwell, F. P., and Hall, William T., Qualitative Analysis, John Wiley & Sons, Inc., New York, NY, Vol. 1, Ninth English Ed., 1937.
Directions for preparing a Jones Reductor may be found in Hillebrand, W. F., et al., Applied Inorganic Analysis, John Wiley & Sons, Inc., New York, NY, Second Ed.,
1953, p. 108.
D1394 − 76 (2014)
FIG. 1 Jones Reduction
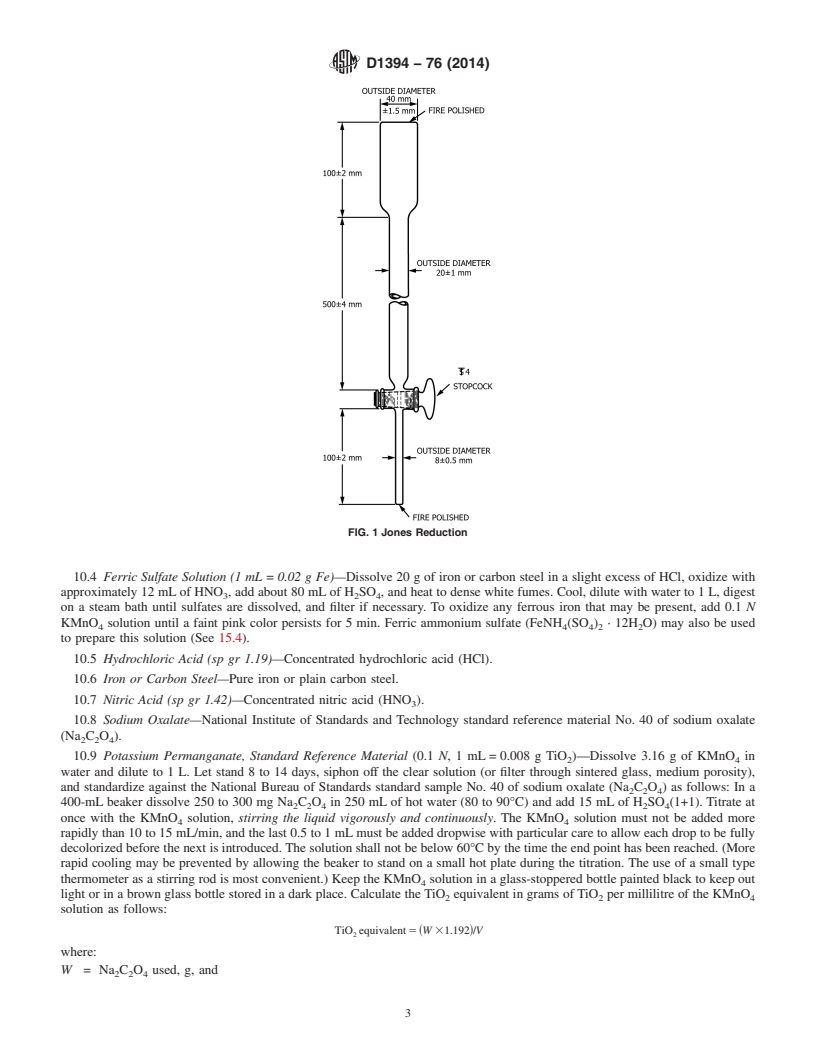
10.4 Ferric Sulfate Solution (1 mL = 0.02 g Fe)—Dissolve 20 g of iron or carbon steel in a slight excess of HCl, oxidize with
approximately 12 mL of HNO , add about 80 mL of H SO , and heat to dense white fumes. Cool, dilute with water to 1 L, digest
3 2 4
on a steam bath until sulfates are dissolved, and filter if necessary. To oxidize any ferrous iron that may be present, add 0.1 N
KMnO solution until a faint pink color persists for 5 min. Ferric ammonium sulfate (FeNH (SO ) · 12H O) may also be used
4 4 4 2 2
to prepare this solution (See 15.4).
10.5 Hydrochloric Acid (sp gr 1.19)—Concentrated hydrochloric acid (HCl).
10.6 Iron or Carbon Steel—Pure iron or plain carbon steel.
10.7 Nitric Acid (sp gr 1.42)—Concentrated nitric acid (HNO ).
10.8 Sodium Oxalate—National Institute of Standards and Technology standard reference material No. 40 of sodium oxalate
(Na C O ).
2 2 4
10.9 Potassium Permanganate, Standard Reference Material (0.1 N, 1 mL = 0.008 g TiO )—Dissolve 3.16 g of KMnO in
2 4
water and dilute to 1 L. Let stand 8 to 14 days, siphon off the clear solution (or filter through sintered glass, medium porosity),
and standardize against the National Bureau of Standards standard sample No. 40 of sodium oxalate (Na C O ) as follows: In a
2 2 4
400-mL beaker dissolve 250 to 300 mg Na C O in 250 mL of hot water (80 to 90°C) and add 15 mL of H SO (1+1). Titrate at
2 2 4 2 4
once with the KMnO solution, stirring the liquid vigorously and continuously. The KMnO solution must not be added more
4 4
rapidly than 10 to 15 mL/min, and the last 0.5 to 1 mL must be added dropwise with particular care to allow each drop to be fully
decolorized before the next is introduced. The solution shall not be below 60°C by the time the end point has been reached. (More
rapid cooling may be prevented by allowing the beaker to stand on a small hot plate during the titration. The use of a small type
thermometer as a stirring rod is most convenient.) Keep the KMnO solution in a glass-stoppered bottle painted black to keep out
light or in a brown glass bottle stored in a dark place. Calculate the TiO equivalent in grams of TiO per millilitre of the KMnO
2 2 4
solution as follows:
TiO equivalent 5 ~W 31.192!/V
where:
W = Na C O used, g, and
2 2 4
D1394 − 76 (2014)
FIG. 2 Jones Reductor, Assembled
V = KMnO solution required for the titration, mL.
10.10 Sulfuric Acid (sp gr 1.84)—Concentrated sulfuric acid (H SO ).
2 4
10.11 Sulfuric Acid (1+1)—Carefully mix 1 volume of H SO (sp gr 1.84) into 1 volume of water with rapid stirring.
2 4
10.12 Sulfuric Acid (1+19)—Carefully mix 1 volume of H SO into 19 volumes of water with rapid stirring.
2 4
11. Procedure
11.1 Determine the dry weight of a weighing bottle and cap to 0.1 mg. Weight to 0.1 mg 300 to 350 mg of the sample to be
analyzed into the weighing bottle.
11.2 Dry the specimen in the opened weighing bottle for 2 h at 105 to 110°C. Cool in a desiccator, cap the bottle, and weigh
as rapidly as possible. Calculate the dry weight of the specimen and use in the actual calculation.
11.3 Transfer the dried specimen to a dry 250 mL chemical- and heat-resistant glass beaker, add 20 mL of H SO (sp gr 1.84)
2 4
and 7 to 8 g of (NH ) SO . Mix well and heat on a hot plate until dense white fumes are evolved, and then continue the heating
4 2 4
over a strong flame until solution is complete (usually requires not over 5 min of boiling) or it is apparent that the residue is
composed of SiO or siliceous matter. Caution should be observed in visually examining this hot solution. Cool the solution, dilute
with 100 mL of water, stir, heat carefully to boiling while stirring, let settle, filter through paper, and transfer the precipitate
completely to the paper.
11.4 Wash the insoluble residue with cold H SO (1+19) until titanium is removed. Dilute the filtrate to 200 mL and add about
2 4
5 mL of NH OH to lower the acidity to approximately 10 to 15 % H SO (by volume). Wash out the Jones reductor with H SO
4 2 4 2 4
(1+19) and water, leaving sufficient water in the reductor to fill to the upper level of the zinc. (These washings should require not
more than one or two drops of 0.1 N KMnO solution to obtain a pink color.) Empty the receiver, and put in it 25 mL of ferric
sulfate solution. Reduce the prepared titanium solution as follows:
11.4.1 Run 50 mL of H SO (1+19) through the reductor at such a uniform rate as to require 5 to 10 min for passage.
2 4
11.4.2 Follow this with the titanium solution at such a uniform rate as to require 10 min to pass through the reductor.
D1394 − 76 (2014)
11.4.3 Wash out with 100 mL of H SO (1+19).
2 4
11.4.4 Finally run through about 100 mL of water. Take care that the reductor is always filled with solution or water to the upper
level of the zinc.
11.5 Gradually release the suction, wash thoroughly the glass tube that was immersed in the ferric sulfate solution, remove the
receiver, and titrate immediately with 0.1 N KMnO solution. Run a blank determination, using the same reagents and washing
the reductor as in the above determination.
12. Calculation
12.1 Calculate the percent of TiO as follows:
~V 2 B! 3T
TiO , %5 3100
S
where:
V = KMnO solution required for titration of specimen, mL
1 4
B = KMnO solution required for titration of the blank, mL
T = TiO equivalent of the KMnO solution, g/mL, and
2 4
S = dried specimen, g.
12.2 The results calculated in accordance with 12.1 will include iron, chromium, arsenic, and any other substance that is reduced
by zinc and acid. However, appreciable quantities of interfering materials are not likely to be encountered in normal, white titanium
pigments.
TOTAL TITANIUM BY THE ALUMINUM
REDUCTION METHOD
13. Scope
13.1 This method gives results similar to those obtained with the Jones Reductor Method (Sections 8 – 12).
14. Apparatus
14.1 Delivery Tube, made of about 4-mm inside diameter glass tubing bent so that there is a horizontal run of about 6 in. (152
mm) and a vertical drop of about 3 in. (76 mm) at one end, and a vertical drop of about 6 in. at the other end.
14.2 Weighing Bottle, wide-mouth, with an external-fitting cap, and no larger than necessary for the required amount of sample.
15. Reagents
15.1 Aluminum Metal Foil, electrolytic grade.
15.2 Ammonium Sulfate—((NH ) SO ).
4 2 4
15.3 Ammonium Thiocyanate Indicator Solution—Dissolve 24.5 g of ammonium thiocyanate (NH CNS) in 80 mL of hot water,
filter, bring to room temperature, and dilute to 100 mL. Keep in a well-stoppered, dark-colored bottle.
15.4 Ferric Ammonium Sulfate Solution (1 mL = 0.005 g TiO )—Dissolve 30.16 g of fresh ferric ammonium sulfate
(FeNH (SO ) · 12H O) in 800 mL of water containing 15 mL of H SO (sp gr 1.84). Add 5 mL of 3 % H O and boil for at least
4 4 2 2 2 4 2 2
15 min then cool to room temperature. Dilute to exactly 1 L and mix well. Filter if cloudy. Standardize using 190 to 210 mg of
NBS standard reference material No. 154 of titanium dioxide and proceeding as directed in Section 16.
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.