ASTM C1108-12
(Test Method)Standard Test Method for Plutonium by Controlled-Potential Coulometry
Standard Test Method for Plutonium by Controlled-Potential Coulometry
SIGNIFICANCE AND USE
4.1 Factors governing selection of a method for the determination of plutonium include available quantity of sample, sample purity, desired level of reliability, and equipment.
4.1.1 This test method determines 5 to 20 mg of plutonium with prior dissolution using Practice C1168.
4.1.2 This test method calculates plutonium concentration in solutions or mass fraction in solids using an electrical calibration based upon Ohm’s Law and the Faraday Constant.
4.1.3 Chemical standards are used for quality control. When prior chemical separation of plutonium is necessary to remove interferences, the quality control standards should be included with each chemical separation batch (9).
4.2 Committee C-26 Safeguards Statement5:
4.2.1 The materials (plutonium metal, plutonium oxide or mixed oxide [(U, Pu) O2] powders and pellets) to which this test method applies are subject to nuclear safeguards regulations governing their possession and use. Materials for use by the commercial nuclear community must also meet compositional specifications.
4.2.2 The analytical method in this test method both meets U. S. Department of Energy guidelines for acceptability of a measurement method for generation of safeguards accountability measurement data and also provides data that may be used to demonstrate specification compliance in buyer-seller interactions.
SCOPE
1.1 This test method describes the determination of dissolved plutonium from unirradiated nuclear-grade (that is, high-purity) materials by controlled-potential coulometry. Controlled-potential coulometry may be performed in a choice of supporting electrolytes, such as 0.9 M HNO3, 1 M HClO4, 1 M HCl, 5 M HCl, and 0.5 M H 2SO4. Limitations on the use of selected supporting electrolytes are discussed in Section 5. Optimum quantities of plutonium for this procedure are 5 to 20 mg.
1.2 Plutonium-bearing materials are radioactive and toxic. Adequate laboratory facilities, such as gloved boxes, fume hoods, controlled ventilation, etc., along with safe techniques must be used in handling specimens containing these materials.
1.3 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: C1108 − 12
Standard Test Method for
1
Plutonium by Controlled-Potential Coulometry
This standard is issued under the fixed designation C1108; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope C1156Guide for Establishing Calibration for a Measure-
ment Method Used toAnalyze Nuclear Fuel Cycle Mate-
1.1 This test method describes the determination of dis-
rials
solved plutonium from unirradiated nuclear-grade (that is,
C1168PracticeforPreparationandDissolutionofPlutonium
high-purity) materials by controlled-potential coulometry.
Materials for Analysis
Controlled-potential coulometry may be performed in a choice
C1210Guide for Establishing a Measurement System Qual-
ofsupporting electrolytes,suchas0.9 MHNO,1 MHClO,1
3 4
ity Control Program for Analytical Chemistry Laborato-
M HCl, 5 M HCl, and 0.5 M H SO . Limitations on the use of
2 4
ries Within the Nuclear Industry
selected supporting electrolytes are discussed in Section 5.
C1297Guide for Qualification of Laboratory Analysts for
Optimumquantitiesofplutoniumforthisprocedureare5to20
the Analysis of Nuclear Fuel Cycle Materials
mg.
E691Practice for Conducting an Interlaboratory Study to
1.2 Plutonium-bearing materials are radioactive and toxic.
Determine the Precision of a Test Method
Adequate laboratory facilities, such as gloved boxes, fume
3. Summary of Test Method
hoods, controlled ventilation, etc., along with safe techniques
mustbeusedinhandlingspecimenscontainingthesematerials.
3.1 In a controlled-potential coulometric measurement, the
substancebeingdeterminedreactsatastationaryelectrode,the
1.3 The values stated in SI units are to be regarded as the
potential of which is maintained at such a value that unwanted
standard. The values given in parentheses are for information
electrode reactions are precluded under the prevailing experi-
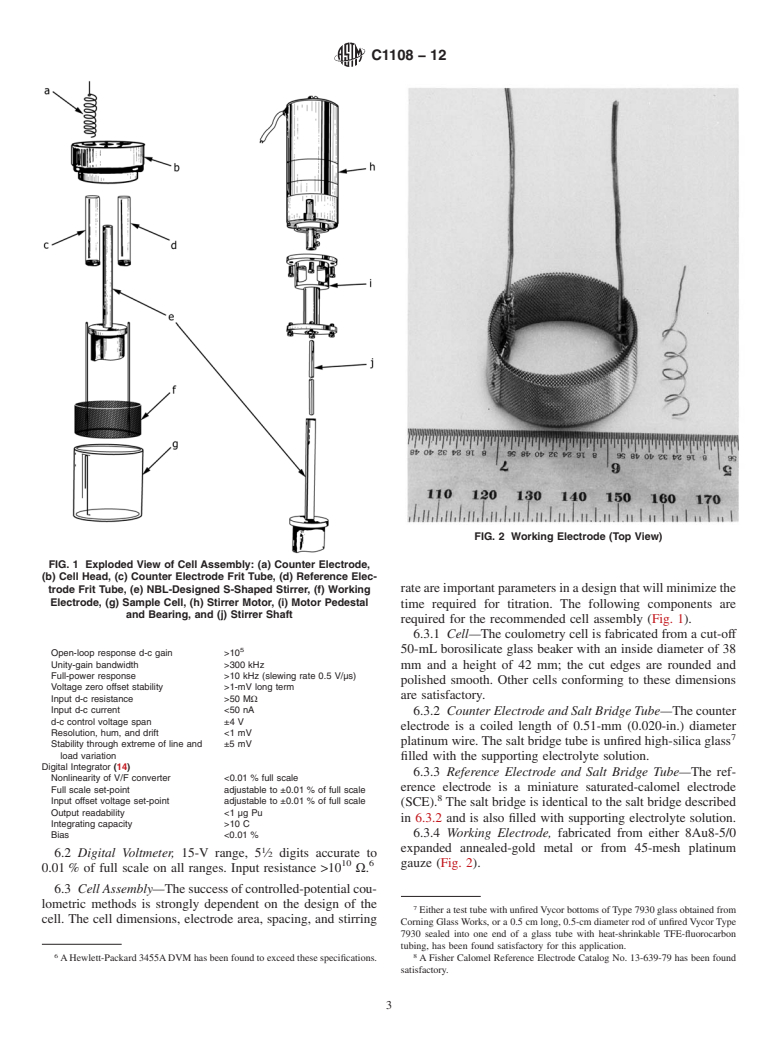
only.
mental conditions. Those substances which have reduction-
1.4 This standard does not purport to address all of the
oxidation (redox) potentials near that of the ion being deter-
safety concerns, if any, associated with its use. It is the
mined constitute interferences. Electrolysis current decreases
responsibility of the user of this standard to establish appro-
exponentially as the reaction proceeds, until constant back-
priate safety and health practices and determine the applica-
ground current is obtained. Detailed discussions of the theory
bility of regulatory limitations prior to use.
andapplicationsofthistechniquehavebeenpublished (1, 2, 3,
3
4, 5, 6). Thecontrol-potentialadjustmenttechnique (7)canbe
2. Referenced Documents
used to terminate the electrolysis of the specimen at constant
2
2.1 ASTM Standards:
background current without exhaustive electrolysis with con-
C859Terminology Relating to Nuclear Materials
siderable reduction in operating time. Use of the control-
C1009Guide for Establishing and Maintaining a Quality
potential adjustment technique requires that the coulometer
AssuranceProgramforAnalyticalLaboratoriesWithinthe
integrator be capable of operations in a bipolar mode and that
Nuclear Industry
the plutonium-containing solution be of high purity, that is,
C1068Guide for Qualification of Measurement Methods by
nuclear grade.
a Laboratory Within the Nuclear Industry
3.2 Plutonium(IV) is reduced to Pu(III) at a working elec-
C1128Guide for Preparation of Working Reference Materi-
trode maintained at a potential more negative than the formal
als for Use in Analysis of Nuclear Fuel Cycle Materials
redox potential. Plutonium(III) is oxidized to Pu(IV) at a
potential more positive than the formal redox potential. The
1
ThistestmethodisunderthejurisdictionofASTMCommitteeC26onNuclear
quantity of plutonium electrolyzed is calculated from the net
Fuel Cycle and is the direct responsibility of Subcommittee C26.05 on Methods of
number of coulombs required for the electrolysis, according to
Test.
Faraday’s law. Corrections for incomplete reaction, derived
CurrenteditionapprovedJuly1,2012.PublishedJuly2012.Originallyapproved
from the Nernst equation, must be applied for electrolysis of
in 1988. Last previous edition approved in 2006 as C1108–99 (2006). DOI:
10.1520/C1108-12.
the sample aliquot (7, 8).
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
the ASTM website. this test method.
Copyright © ASTM International, 100 Barr Harbor Drive, P
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: C1108 − 99 (Reapproved 2006) C1108 − 12
Standard Test Method for
1
Plutonium by Controlled-Potential Coulometry
This standard is issued under the fixed designation C1108; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes the determination of plutonium in solutions of dissolved plutonium from unirradiated
nuclear-grade (that is, high-purity) materials by controlled-potential coulometry. Controlled-potential coulometry may be
performed in a choice of supporting electrolytes, such as 0.9 M HNO , 1 M HClO , 1 M HCl, 5 M HCl, and 0.5 M H SO .
3 4 2 4
Limitations on the use of selected supporting electrolytes are discussed in Section 5. Optimum quantities of plutonium for this
procedure are 5 to 1020 mg.
1.2 Plutonium-bearing materials are radioactive and toxic. Adequate laboratory facilities, such as gloved boxes, fume hoods,
controlled ventilation, etc., along with safe techniques must be used in handling specimens containing these materials.
1.3 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
C859 Terminology Relating to Nuclear Materials
C1009 Guide for Establishing and Maintaining a Quality Assurance Program for Analytical Laboratories Within the Nuclear
Industry
C1068 Guide for Qualification of Measurement Methods by a Laboratory Within the Nuclear Industry
C1128 Guide for Preparation of Working Reference Materials for Use in Analysis of Nuclear Fuel Cycle Materials
C1156 Guide for Establishing Calibration for a Measurement Method Used to Analyze Nuclear Fuel Cycle Materials
C1168 Practice for Preparation and Dissolution of Plutonium Materials for Analysis
C1210 Guide for Establishing a Measurement System Quality Control Program for Analytical Chemistry Laboratories Within
the Nuclear Industry
C1297 Guide for Qualification of Laboratory Analysts for the Analysis of Nuclear Fuel Cycle Materials
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3. Summary of Test Method
3.1 In a controlled-potential coulometric measurement, the substance being determined reacts at an a stationary electrode, the
potential of which is maintained at such a value that unwanted electrode reactions are precluded under the prevailing experimental
conditions. Those substances which have reduction-oxidation (redox) potentials near that of the ion being determined constitute
interferences. Electrolysis current decreases exponentially as the reaction proceeds, until constant background current is obtained.
3
Detailed discussions of the theory and applications of this technique have been published (1, 2, 3, 4, 5, 6). The control-potential
adjustment technique (7) can be used to terminate the electrolysis of the specimen at constant background current without
exhaustive electrolysis with considerable reduction in operating time. Use of the control-potential adjustment technique requires
that the coulometer integrator be capable of operations in a bipolar mode and that the plutonium-containing solution be of high
purity, that is, nuclear grade.
1
This test method is under the jurisdiction of ASTM Committee C26 on Nuclear Fuel Cycle and is the direct responsibility of Subcommittee C26.05 on Methods of Test.
Current edition approved July 1, 2006July 1, 2012. Published October 2006July 2012. Originally approved in 1988. Last previous edition approved in 19992006 as
C1108 – 99.C1108 – 99 (2006). DOI: 10.1520/C1108-99R06.10.1520/C1108-12.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The boldface numbers in parentheses refer to the list of references at the end of this test method.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, P
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:C1108–99(Reapproved2006) Designation: C1108 – 12
Standard Test Method for
1
Plutonium by Controlled-Potential Coulometry
This standard is issued under the fixed designation C1108; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes the determination of dissolved plutonium in solutions offrom unirradiated nuclear-grade (that is,
high-purity) materials by controlled-potential coulometry. Controlled-potential coulometry may be performed in a choice of
supportingelectrolytes,suchas0.9MHNO ,1MHClO ,1MHCl,5MHCl,and0.5MH SO .Limitationsontheuseofselected
3 4 2 4
supporting electrolytes are discussed in Section 5. Optimum quantities of plutonium for this procedure are 5 to 1020 mg.
1.2 Plutonium-bearing materials are radioactive and toxic. Adequate laboratory facilities, such as gloved boxes, fume hoods,
controlled ventilation, etc., along with safe techniques must be used in handling specimens containing these materials.
1.3 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
C859 Terminology Relating to Nuclear Materials
C1009 GuideforEstablishingaQualityAssuranceProgramforAnalyticalChemistryLaboratoriesWithintheNuclearIndustry
C1068 Guide for Qualification of Measurement Methods by a Laboratory Within the Nuclear Industry
C1128 Guide for Preparation of Working Reference Materials for Use in Analysis of Nuclear Fuel Cycle Materials
C1156 Guide for Establishing Calibration for a Measurement Method Used to Analyze Nuclear Fuel Cycle Materials
C1168 Practice for Preparation and Dissolution of Plutonium Materials for Analysis
C1210 Guide for Establishing a Measurement System Quality Control Program forAnalytical Chemistry Laboratories Within
the Nuclear Industry
C1297 Guide for Qualification of Laboratory Analysts for the Analysis of Nuclear Fuel Cycle Materials
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3. Summary of Test Method
3.1 In a controlled-potential coulometric measurement, the substance being determined reacts at a stationary electrode, the
potentialofwhichismaintainedatsuchavaluethatunwantedelectrodereactionsareprecludedundertheprevailingexperimental
conditions. Those substances which have reduction-oxidation (redox) potentials near that of the ion being determined constitute
interferences. Electrolysis current decreases exponentially as the reaction proceeds, until constant background current is obtained.
3
Detailed discussions of the theory and applications of this technique have been published (1, 2, 3, 4, 5, 6). The control-potential
adjustment technique (7) can be used to terminate the electrolysis of the specimen at constant background current without
exhaustive electrolysis with considerable reduction in operating time. Use of the control-potential adjustment technique requires
that the coulometer integrator be capable of operations in a bipolar mode and that the plutonium-containing solution be of high
purity, that is, nuclear grade.
3.2 Plutonium(IV) is reduced to Pu(III) at a working electrode maintained at a potential more negative than the formal redox
potential. Plutonium(III) is oxidized to Pu(IV) at a potential more positive than the formal redox potential. The quantity of
plutonium electrolyzed is calculated from the net number of coulombs required for the electrolysis, according to Faraday’s law.
Corrections for incomplete reaction, derived from the Nernst equation, must be applied for electrolysis of the sample aliquot (7,
8).
1
This test method is under the jurisdiction ofASTM Committee C26 on Nuclear Fuel Cycle and is the direct responsibility of Subcommittee C26.05 on Methods ofTest.
Current edition approved July 1, 2006.2012. Published October 2006.July 2012. Originally approved in 1988. Last previous edition approved in 19992006 as C1108–99
(2006). DOI: 10.1520/C1108-99R0612.
2
ForreferencedASTMstandards,visit
...











Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.