ASTM D4763-88(2001)
(Practice)Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy
Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy
SCOPE
1.1 This practice allows for the identification of 90 chemicals that may be found in water or in surface layers on water. This practice is based on the use of room-temperature fluorescence spectra taken from lists developed by the U.S. Environmental Protection Agency and the U.S. Coast Guard (1). Ref (1) is the primary source for these spectra. This practice is also based on the assumption that such chemicals are either present in aqueous solution or are extracted from water into an appropriate solvent.
1.2 Although many organic chemicals containing aromatic rings, heterocyclic rings, or extended conjugated double-bond systems have appreciable quantum yields of fluorescence, this practice is designed only for the specific compounds listed. If present in complex mixtures, preseparation by high-performance liquid chromatography (HPLC), column chromatography, or thin-layer chromatography (TLC) would probably be required.
1.3 If used with HPLC, this practice could be used for the identification of fluorescence spectra generated by optical multichannel analyzers (OMA) or diode-array detectors.
1.4 For simple mixtures, or in the presence of other nonfluorescing chemicals, separatory techniques might not be required. The excitation and emission maximum wavelengths listed in this practice could be used with standard fluorescence techniques Refs (2-6) to quantitate these ninety chemicals once identification had been established. For such uses, generation of a calibration curve, to determine the linear range for use of fluorescence quantitation would be required for each chemical. Examination of solvent blanks to subtract or eliminate any fluorescence background would probably be required.
1.5 This standard does not purport to address the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 4763 – 88 (Reapproved 2001)
Standard Practice for
Identification of Chemicals in Water by Fluorescence
Spectroscopy
This standard is issued under the fixed designation D 4763; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This practice allows for the identification of 90 chemi- 2.1 ASTM Standards:
cals that may be found in water or in surface layers on water. D 1129 Terminology Relating to Water
This practice is based on the use of room-temperature fluores- D 1193 Specification for Reagent Water
cence spectra taken from lists developed by the U.S. Environ- E 131 Terminology Relating to Molecular Spectroscopy
mental Protection Agency and the U.S. Coast Guard (1). Ref E 275 Practice for Describing and Measuring Performance
(1) is the primary source for these spectra. This practice is also of Ultraviolet, Visible, and Near Infrared Spectrophotom-
based on the assumption that such chemicals are either present eters
in aqueous solution or are extracted from water into an
3. Terminology
appropriate solvent.
3.1 Definitions—For definitions of terms used in this prac-
1.2 Although many organic chemicals containing aromatic
rings, heterocyclic rings, or extended conjugated double-bond tice, refer to Terminology D 1129, Specification D 1193, and
definitions under the jurisdiction of Committee E-13 such as
systems have appreciable quantum yields of fluorescence, this
practice is designed only for the specific compounds listed. If Definitions E 131 and Practice E 275.
present in complex mixtures, preseparation by high-
4. Summary of Practice
performance liquid chromatography (HPLC), column chroma-
4.1 This practice uses well tested fluorescence techniques to
tography, or thin-layer chromatography (TLC) would probably
detect and identify (or determine the absence of) 90 chemicals
be required.
that have relatively high fluorescence yields. Table 1 lists for
1.3 If used with HPLC, this practice could be used for the
each chemical an appropriate solvent (either cyclohexane,
identification of fluorescence spectra generated by optical
water, methyl or ethyl alcohol, depending on solubility), a
multichannel analyzers (OMA) or diode-array detectors.
suggested excitation wavelength for maximum sensitivity, a
1.4 For simple mixtures, or in the presence of other non-
wavelength corresponding to the emission maximum, the
fluorescing chemicals, separatory techniques might not be
number of fluorescence peaks and shoulders, the width (full
required. The excitation and emission maximum wavelengths
width at half of the maximum emission intensity) of the
listed in this practice could be used with standard fluorescence
strongest fluorescence peak and the detection limit for the
techniques Refs (2-6) to quantitate these ninety chemicals once
experimental conditions given. Detection limits could be low-
identification had been established. For such uses, generation
ered, following identification, by using broader slit widths. A
of a calibration curve, to determine the linear range for use of
listofcorrectedfluorescencespectraforthechemicalsincluded
fluorescence quantitation would be required for each chemical.
in this practice are also available (1).
Examination of solvent blanks to subtract or eliminate any
4.2 Identification of the sample is made by comparison of
fluorescence background would probably be required.
the obtained spectra with information in Table 1 and by direct
1.5 This standard does not purport to address the safety
visual comparison of appropriate spectra with positions of
concerns, if any, associated with its use. It is the responsibility
principal peaks in agreement to 62 nm and ratios of peak
of the user of this standard to establish appropriate safety and
heights in agreement to 610 % if corrected spectrofluorom-
health practices and determine the applicability of regulatory
eters are used.
limitations prior to use.
4.3 Spectral distortions due to self-absorption or fluores-
cence quenching or dimer formation may occur at higher
concentrations (for example, 100 ppm or µg/mL). If this is
This practice is under the jurisdiction of ASTM Committee D19 on Water and
is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
Organic Substances in Water.
Current edition approved July 7, 1988. Published September 1988.
2 3
The boldface numbers in parentheses refer to the list of references at the end of Annual Book of ASTM Standards, Vol 11.01.
this practice. Annual Book of ASTM Standards, Vol 03.06.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 4763 – 88 (2001)
suspected, the solution should be diluted and additional fluo- willbeusefulfordistinguishingsinglefluorescentchemicalsin
rescence spectra generated. If a suspected chemical is not solution, simple mixtures or single fluorescing chemicals in the
detected on excitation at the appropriate wavelength, it usually presence of other nonfluorescing chemicals. Chemicals with
can be assumed that it is not present above the detection limit, high fluorescence yields tend to have aromatic rings, some
barring interference effects due to absorption or quenching that heterocyclic rings or extended conjugated double-bond sys-
can usually be anticipated. tems.Typical chemicals included on this list include aromatics,
substituted aromatics such as phenols, polycyclic aromatic
5. Significance and Use
hydrocarbons (PAH’s), some pesticides such as DDT, poly-
5.1 This practice is useful for detecting and identifying (or chlorinated biphenyls (PCB’s), some heterocyclics, and some
determining the absence of) 90 chemicals with relatively high esters, organic acids, and ketones.
fluorescenceyields(seeTable1).Mostcommonly,thispractice
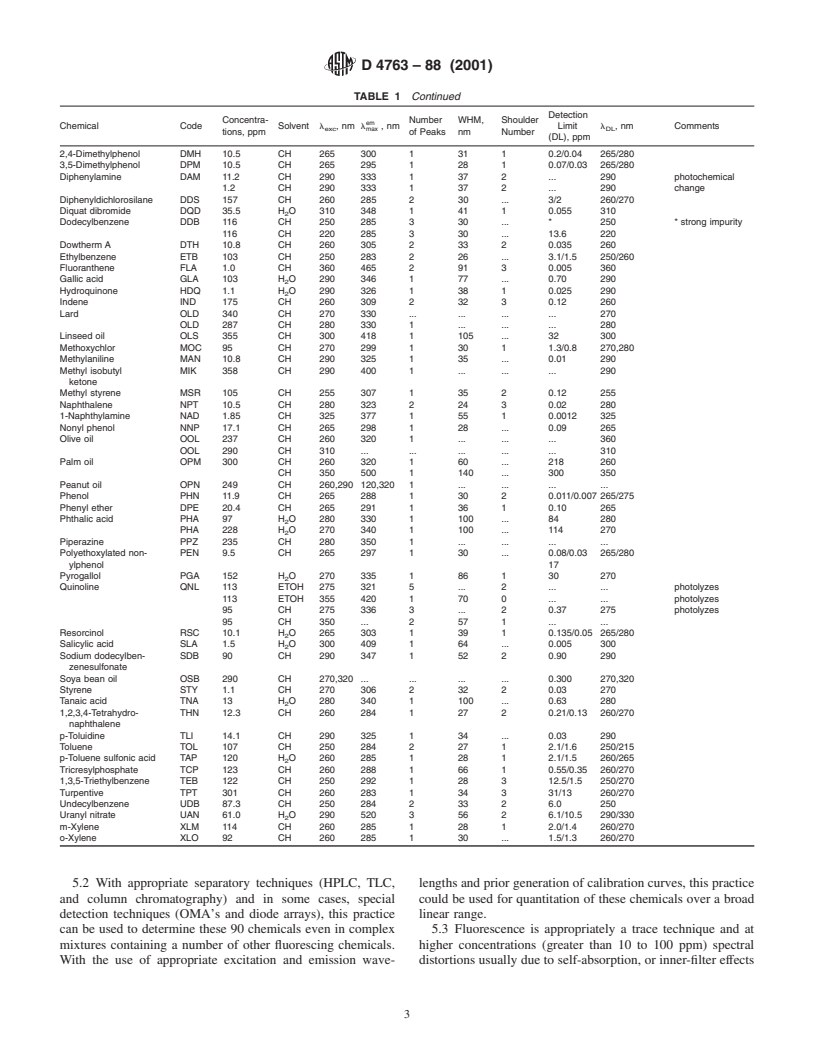
TABLE 1 Summary of Experimental Parameters and Results
Detection
Concentra- Number WHM, Shoulder
em
Chemical Code Solvent l ,nm l ,nm Limit l , nm Comments
exc max DL
tions, ppm of Peaks nm Number
(DL), ppm
Acenaphthene ACN 1.03 CH 290 323 4 . 3 0.001 290
Acetone ACT 227 CH 290 410 1 . . 212 290
Acridine ACR 96 CH 285/355 386/422 4/2 . 2/0 . .
ACR 9.6 ETOH 290/355 357/415 2/2 . 1/1 0.02/0.04 290/355
Aniline ANL 15.5 CH 280 316 1 . . 0.037 280
Anthracene ATH 1.03 CH 355 378 4 . 1 0.001 355
ATH 1.55 ETOH 355 380 4 . 1 0.001 355
Aroclor 1242 PC4 131 CH 270 317 2 35 1 0.3 270
1254 PC5 129 CH 270 317 2 36 1 2 270
Atrazine ATZ 369 CH 290 350 1 . . 300 290
Azinphosmethyl AZP 112 CH 350 410 2 60 . 10 350
AZP 122 ETOH 340 420 2 80 . 4 340
Benz(a)anthracene BAT 1.1 CH 280 386 4 . 1 0.003 280
Benzene BNZ 79 CH 250 279 3 24 1 2/4 250/265
Benzonitrile BZN 9.9 CH 260 287 2 28 1 0.1/0.1 260/270
Benzo(a)pyrene BAP 0.088 CH 370 405 6 . 2 0.002 370
Benzyl alcohol BAL 99 CH 250 284 2 27 1 0.1/0.1 250/260
Benzyl amine BZM 118 CH 250 283 1 27 2 3/2 250/260
Benzyl triethylam- BMA 210 H O 250 280 1 28 . 59 250
monium chloride
Bisphenol A BPA 10.5 ETOH 270 304 1 30 1 0.04/0.02 270/285
Brucine BRU 13.5 ETOH 280 327 1 56 . 2/2 280/295
O-tert-Butylphenol BOP 21 CH 265 295 1 30 1 0.1/0.1 265/275
p-tert-Butylphenol BTP 17.5 CH 260 295 1 31 1 0.6/0.4 260/280
Carbaryl CBY 1.0 CH 285 335 2 36 2 0.01 285
Carnauba wax WCA 63.5 CH 260 310 1 64 . 42 260
Castor oil OCA 390 ETOH 290 328 1 43 2 20 290
OCA 286 CH 280/320 . 1 . . 180/300 280/320
Catechol CTC 8.7 H O 265 310 1 46 . 0.4/0.2 265/280
4-Chloroaniline CAP 17.2 CH 290 328 1 36 1 0.2 290
1-Chloronaphthalene CNA 11.3 CH 290 328 3 34 4 0.1 290
p-Chlorophenol CPN 101 CH 260 305 1 30 . 1/0.1 260/285
Chlorpyrifos (Duraban) DUR 25.3 CH 280 326 1 52 . 1/0.5 280/295
p-Chlorotoluene CTN 23.8 CH 265 288 1 29 3 1/0.8 265/275
p-Chloro-o-toluidine COT 25 CH 290 328 1 39 1 0.09 300
Chrysene CRY 1.0 CH 270 383 5 . . 0.002 270
Coconut oil OCC 286 CH 290 330 . . . 100 290
Cod liver oil OCL 323 CH 260/280 320/320 1/1 150 . 260,140 260,280
330 500 1 65 330
Copper naphthenate CNN 98 CH 260 326 1 60 3 3/1 260/280
Cottonseed oil OCS 305 CH 280/320 320/380 . . . 165,300 280,320
Coumaphos COU 11.4 CH 320 377 1 74 . 0.3 320
o-Cresol CRO 12.0 CH 265 293 1 30 1 0.04 280
p-Cresol CRP 10.3 CH 265 299 1 30 . 0.03 280
Cumene CUM 101 CH 250 283 2 28 1 3 250
p-Cymene CMP 11.8 CH 260 285 1 28 2 0.4/0.2 260/270
DDD DDD 61.0 CH 240 294 1 30 2 4 240
DDT DDT 87 CH 245 291 2 28 2 7 245
1,2,5,6-Dibenzanthracene DBA 0.015 CH 300 396 4 . 2 0.001 300
Dicamba DIC 22.2 H O 310 420 1 70 . 0.9 310
Dichlorobenil DIB 108 CH 285 312 1 30 . 0.6 285
2,4-Dichlorophenoxy- DCA 159 CH 270 310 1 46 1 30 270
acetic acid
Diethylbenzene DEB 100 CH 255 283 1 28 2 0.2/0.1 255/270
Diethylene glycol DEG 202 CH 265 310 2 . . 202 265
Diethylphthalate DEP 145/289 CH 260/280 300/320 1/1 . . . 280
D 4763 – 88 (2001)
TABLE 1 Continued
Detection
Concentra- Number WHM, Shoulder
em
Chemical Code Solvent l ,nm l ,nm Limit l , nm Comments
exc max DL
tions, ppm of Peaks nm Number
(DL), ppm
2,4-Dimethylphenol DMH 10.5 CH 265 300 1 31 1 0.2/0.04 265/280
3,5-Dimethylphenol DPM 10.5 CH 265 295 1 28 1 0.07/0.03 265/280
Diphenylamine DAM 11.2 CH 290 333 1 37 2 . 290 photochemical
1.2 CH 290 333 1 37 2 . 290 change
Diphenyldichlorosilane DDS 157 CH 260 285 2 30 . 3/2 260/270
Diquat dibromide DQD 35.5 H O 310 348 1 41 1 0.055 310
Dodecylbenzene DDB 116 CH 250 285 3 30 . * 250 * strong impurity
116 CH 220 285 3 30 . 13.6 220
Dowtherm A DTH 10.8 CH 260 305 2 33 2 0.035 260
Ethylbenzene ETB 103 CH 250 283 2 26 . 3.1/1.5 250/260
Fluoranthene FLA 1.0 CH 360 465 2 91 3 0.005 360
Gallic acid GLA 103 H O 290 346 1 77 . 0.70 290
Hydroquinone HDQ 1.1 H O 290 326 1 38 1 0.025 290
Indene IND 175 CH 260 309 2 32 3 0.12 260
Lard OLD 340 CH 270 330 . . . . 270
OLD 287 CH 280 330 1 . . . 280
Linseed oil OLS 355 CH 300 418 1 105 . 32 300
Methoxychlor MOC 95 CH 270 299 1 30 1 1.3/0.8 270,280
Methylaniline MAN 10.8 CH 290 325 1 35 . 0.01 290
Methyl isobutyl MIK 358 CH 290 400 1 . . . 290
ketone
Methyl styrene MSR 105 CH 255 307 1 35 2 0.12 255
Naphthalene NPT 10.5 CH 280 323 2 24 3 0.02 280
1-Naphthylamine NAD 1.85 CH 325 377 1 55 1 0.0012 325
Nonyl phenol NNP 17.1 CH 265 298 1 28 . 0.09 265
Olive oil OOL 237 CH 260 320 1 . . . 360
OOL 290 CH 310 . . . . . 310
Palm oil OPM 300 CH 260 320 1 60 . 218 260
CH 350 500 1 140 . 300 350
Peanut oil OPN 249 CH 260,290 120,320 1 . . . .
Phenol PHN 11.9 CH 265 288 1 30 2 0.011/0.007 265/275
Phenyl ether DPE 20.4 CH 265 291 1 36 1 0.10 265
Phthalic acid PHA 97 H O 280 330 1 100 . 84 280
PHA 228 H O 270 340 1 100 . 114 270
Piperazine PPZ 235 CH 280 350 1 . . . .
Polyethoxylated non- PEN 9.5 CH 265 297 1 30 . 0.08/0.03 265/280
ylphenol 17
Pyrogallol PGA 152 H O 270 335 1 86 1 30 270
Quinoline QNL 113 ETOH 275 321 5 . 2 . . photolyzes
113 ETOH 355 420 1 70 0 . . photolyzes
95 CH 275 336 3 . 2 0.37 275 photolyzes
95 CH 350 . 2 57 1 . .
Resorcinol RSC 10.1 H O 265 303 1 39 1 0.135/0.05 265/280
Salicylic acid SLA 1.5 H O 300 409 1 64 . 0.005 300
Sodium dodecylben- SDB 90 CH 290 347 1 52 2 0.90 290
zenesulfonate
Soya bean oil OSB 290 CH 270,320 . . . . 0.300 270,320
Styrene STY 1.1 CH 270 306 2 32 2 0.03 270
Tanaic acid TNA 13 H O 280 340 1 100 . 0.63 280
1,2,3,4-Tetrahydro- THN 12.3 CH 260 284 1 27 2 0.21/0.13 260/270
naphthalene
p-Toluidine TLI 14.1 CH 290 325 1 34 . 0.03 290
Toluene TOL 107 CH 250 284 2 27 1 2.1/1.6 250/215
p-Toluene sulfonic acid TAP 120 H O 260 285 1 28 1 2.1/1.5 260/265
Tricresylphosphate TCP 123 CH 260 288 1 66 1 0.55/0.35 260/270
1,3,5-Triethylbenzene TEB 122 CH 250 292 1 28 3 12.5/1.5 250/270
Turpentive TPT 301 CH 260 283 1 34 3 31/13 260/270
Undecylbenzene UDB 87.3 CH 250 284 2 33 2 6.0 250
Uranyl nitrate UAN 61.0 H O 290 520 3 56 2 6.1/10.5 290/330
m-Xylene XLM 114 CH 260 285 1 28 1 2.0/1.4 260/270
o-Xylene XLO 92 CH 260 285 1 30 . 1.5/1.3 260/270
5.2 With appropriate separatory techniques (HPLC, TLC, lengths and prior generation of calibration curves, this practice
and column chromatography) and in some cases, special could be used for quantitation of these chemicals over a broad
detection techniques (OMA’s and diode arrays), this practice linear range.
can be used to determine these 90 chemicals even in complex 5.3 Fluorescence is appropriately a trace technique and at
mixtures containing a number of other fluorescing chemicals. higher concentrations (greater than 10 to 100 ppm) spectral
With the use of appropriate excitation and emission wave- distortions usually due to self-absorption, or inner-filter effects
D 4763 – 88 (2001)
but sometimes ascribed to fluorescence quenching, may be 8.2 Purity of Water— Unless otherwise indicated, refer-
observed. These effects can usually be eliminated by diluting ences to water shall be understood to mean Type II reagent
the solution. Detection limits can be lowered following iden- water conforming to Specification D 1193. Check the water
tification by using broader slit widths, but this may result in purity by running water blanks.
spectral broadening and distortion.
9. Sampling and Sample Preparation
5.4 This practice assumes the use of a corrected spectrof-
9.1 Neat samples (from a surface film or layer on water)
luorometer (that is, one capable of producing corrected fluo-
only require dilution in an appropriate solvent (after skimming
rescence spectra). On an uncorrected instrument, peak shifts
from the surface of the water using perforated TFE-
and spectral distortions and changes in peak ratios may be
fluorocarbon if on water). An initial concentration for an
noted. An uncorrected spectrofluorometer can also be used if
unknown might be 100 µg/mL for preferably 25 mL of
appropriate data is generated on the instrument to be used.
solution, with further dilutions once a fluorescence signal
detected, down to 10 or 1 µg/mL. If a particular compound is
6. Interferences
notsolubleincyclohexane,thefollowingsolventsmaybetried
6.1 For the identification of compounds with low fluores-
in order: water, methanol, ethanol, and acetonitrile.
cence yields and relatively high detection limits, the presence
9.2 If an unknown is dissolved in water (assuming no
ofotherchemicalswithhighfluorescenceyieldsemittinginthe
chemicals such as humic acid are present at levels that might
samespectralregion,forexample,
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.