ASTM E3244-23
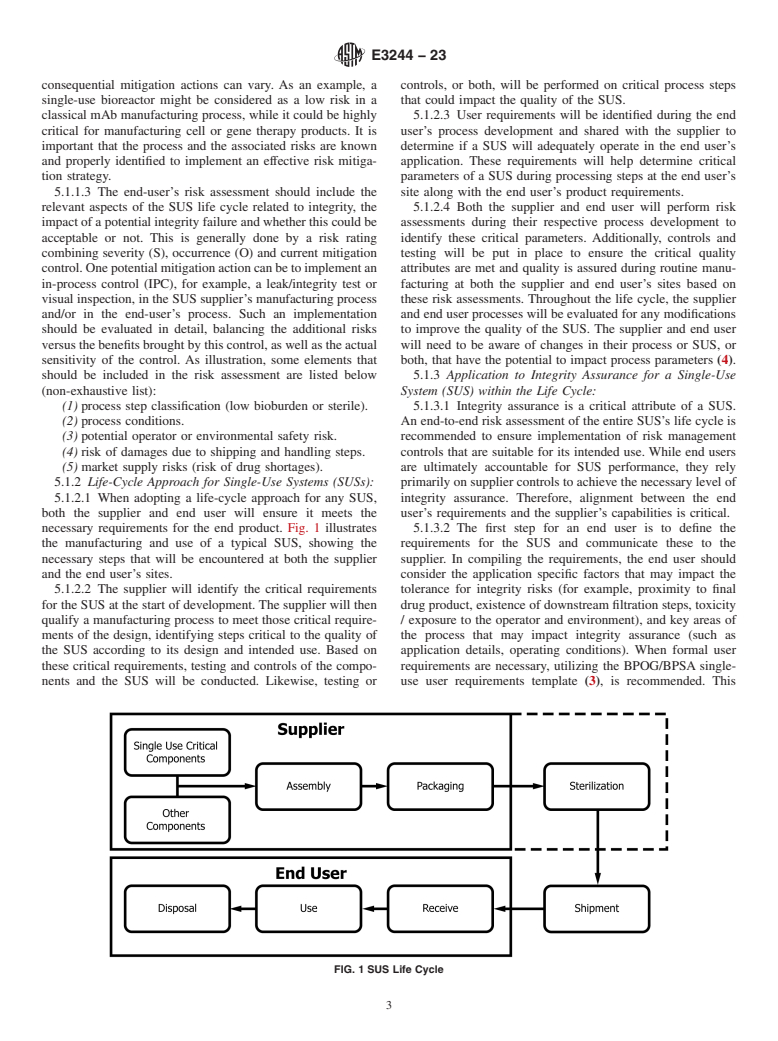
(Practice)Standard Practice for Integrity Assurance and Testing of Single-Use Systems
Standard Practice for Integrity Assurance and Testing of Single-Use Systems
SIGNIFICANCE AND USE
4.1 This practice provides:
4.1.1 A holistic approach to evaluate risks associated with an integrity breach in a SUS, considering its life cycle from development to disposal.
4.1.2 An overview of physical and microbial test methods that could be applicable to SUS testing, for qualification and validation purposes, as well as for routine testing.
4.1.3 Information on the main challenges faced when testing SUSs for integrity.
4.2 This practice can be used by SUS suppliers and SUS end users to define an integrity assurance strategy for SUSs, with the relevant tests when appropriate.
SCOPE
1.1 This practice uses quality risk management (QRM) and life-cycle approach to establish integrity assurance of single-use systems (SUSs), such as but not limited to bag assemblies and liquid transfer sets for processing, storage, and shipping of (bio)pharmaceutical products. It gives recommendations to identify failure modes and risks associated with such systems and their use-cases and how to identify the relevant leak(s) of concern. Integrity assurance in this context is limited to the barrier properties of the SUS, linked to microbial integrity and bioburden control (product quality) and liquid product loss (operator and environmental contamination). The required level of integrity assurance will depend on how critical the application is and can be interpreted in different ways. It can also vary between processes and applications used for different modalities (for example, advanced therapies). Other package barrier properties different from that, such as but not limited to gas barrier properties for gas headspace preservation, as well as porous barrier packages are not considered. Specific aspects how to address the contamination control strategy (CCS) for SUS are also described in chapters 8.131ff of the new Revision of Annex 1 (1),2 including chapter 8.137 regarding SUS integrity.
1.2 The test method overview provides descriptions that focus on the standard test setup and the identification of challenges in combination with SUSs. Details, including specific test setups, test parameter, and result interpretation, are not discussed. For more detailed information refer to Test Method E3251 for microbial test methods, and to Test Method E3336 for physical test methods.
1.3 This practice is not intended to apply to the use of single-use technology for primary containers, combination products (products composed of any combination of a drug, device, or biological product), or devices. Appropriate procedures related to these products are discussed in documents covering the integrity assurance for primary containers (2) or medical products (1, 3).
1.4 Techniques and procedures for complaint management and root cause analysis related to integrity failures are also not discussed.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E3244 − 23
Standard Practice for
1
Integrity Assurance and Testing of Single-Use Systems
This standard is issued under the fixed designation E3244; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope covering the integrity assurance for primary containers (2) or
medical products (1, 3).
1.1 This practice uses quality risk management (QRM) and
life-cycle approach to establish integrity assurance of single- 1.4 Techniques and procedures for complaint management
use systems (SUSs), such as but not limited to bag assemblies and root cause analysis related to integrity failures are also not
and liquid transfer sets for processing, storage, and shipping of discussed.
(bio)pharmaceutical products. It gives recommendations to
1.5 The values stated in SI units are to be regarded as
identify failure modes and risks associated with such systems
standard. No other units of measurement are included in this
and their use-cases and how to identify the relevant leak(s) of
standard.
concern. Integrity assurance in this context is limited to the
1.6 This standard does not purport to address all of the
barrier properties of the SUS, linked to microbial integrity and
safety concerns, if any, associated with its use. It is the
bioburden control (product quality) and liquid product loss
responsibility of the user of this standard to establish appro-
(operator and environmental contamination). The required
priate safety, health, and environmental practices and deter-
level of integrity assurance will depend on how critical the
mine the applicability of regulatory limitations prior to use.
application is and can be interpreted in different ways. It can
1.7 This international standard was developed in accor-
also vary between processes and applications used for different
dance with internationally recognized principles on standard-
modalities (for example, advanced therapies). Other package
ization established in the Decision on Principles for the
barrier properties different from that, such as but not limited to
Development of International Standards, Guides and Recom-
gas barrier properties for gas headspace preservation, as well as
mendations issued by the World Trade Organization Technical
porous barrier packages are not considered. Specific aspects
Barriers to Trade (TBT) Committee.
how to address the contamination control strategy (CCS) for
SUS are also described in chapters 8.131ff of the new Revision
2. Referenced Documents
2
of Annex 1 (1), including chapter 8.137 regarding SUS
3
2.1 ASTM Standards:
integrity.
E3051 Guide for Specification, Design, Verification, and
1.2 The test method overview provides descriptions that
Application of Single-Use Systems in Pharmaceutical and
focus on the standard test setup and the identification of
Biopharmaceutical Manufacturing
challenges in combination with SUSs. Details, including spe-
E3251 Test Method for Microbial Ingress Testing on Single-
cific test setups, test parameter, and result interpretation, are
Use Systems
not discussed. For more detailed information refer to Test
E3336 Test Method for Physical Integrity Testing of Single-
Method E3251 for microbial test methods, and to Test Method
Use Systems
E3336 for physical test methods.
4
2.2 ICH Documents:
1.3 This practice is not intended to apply to the use of
ICH Q9(R1) Quality Risk Management
single-use technology for primary containers, combination
products (products composed of any combination of a drug,
3. Terminology
device, or biological product), or devices. Appropriate proce-
3.1 Definitions:
dures related to these products are discussed in documents
1 3
This practice is under the jurisdiction of ASTM Committee E55 on Manufac- For referenced ASTM standards, visit the ASTM website, www.astm.org, or
ture of Pharmaceutical and Biopharmaceutical Products and is the direct responsi- contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
bility of Subcommittee E55.07 on Single Use Systems. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved June 1, 2023. Published August 2023. Originally the ASTM website.
4
approved in 2020. Last previous edition approved in 2020 as E3244 – 20. DOI: Available from International Conference on Harmonisation of Technical
10.1520/E3244-23. Requirements for Registration of Pharmaceuticals for Human Use (ICH), ICH
2
The boldface numbers in parentheses
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E3244 − 20 E3244 − 23
Standard Practice for
1
Integrity Assurance and Testing of Single-Use Systems
This standard is issued under the fixed designation E3244; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice uses quality risk management (QRM) and life-cycle approach to establish integrity assurance of single-use
systems (SUSs), such as but not limited to bag assemblies and liquid transfer sets for processing, storage, and shipping of
(bio)pharmaceutical products. It gives recommendations to identify failure modes and risks associated with such systems and their
use-cases and how to identify the relevant leak(s) of concern. Integrity assurance in this context is limited to the barrier properties
of the SUS, linked to microbial integrity and bioburden control (product quality) and liquid product loss (operator and
environmental contamination). The required level of integrity assurance will depend on how critical the application is and can be
interpreted in different ways. It can also vary between processes and applications used for different modalities (for example,
advanced therapies). Other package barrier properties different from that, such as but not limited to gas barrier properties for gas
headspace preservation, as well as porous barrier packages are not considered. Specific aspects how to address the contamination
2
control strategy (CCS) for SUS are also described in chapters 8.131ff of the new Revision of Annex 1 (1), including chapter 8.137
regarding SUS integrity.
1.2 The test method overview provides descriptions that focus on the standard test setup and the identification of challenges in
combination with SUSs. Details, including specific test setups, test parameter, and result interpretation, are not discussed. For more
detailed information refer to Test Method E3251 for microbial test methods, and to Test Method E3336 for physical test methods.
1.3 This practice is not intended to apply to the use of single-use technology for primary containers, combination products
(products composed of any combination of a drug, device, or biological product), or devices. Appropriate procedures related to
these products are discussed in documents covering the integrity assurance for primary containers (12) or medical products (21,
3).
1.4 Techniques and procedures for complaint management and root cause analysis related to integrity failures are also not
discussed.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
1
This practice is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.04 on General Biopharmaceutical Standards.
Current edition approved Feb. 1, 2020June 1, 2023. Published April 2020August 2023. Originally approved in 2020. Last previous edition approved in 2020 as E3244 – 20.
DOI: 10.1520/E3244-20.10.1520/E3244-23.
2
The boldface numbers in parentheses refer to a list of references at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E3244 − 23
2. Referenced Documents
3
2.1 ASTM Standards:
E3051 Guide for Specification, Design, Verification, and Application of Single-Use Systems in Pharmaceutical and Biophar-
maceutical Manufacturing
F2095E3251 Test Methods for Pressure Decay Leak Test for Flexible Packages With and Without Restraining PlatesMethod for
Microbial Ingress Testing on Single-Use Systems
F2391E3336 Test Method for Measur
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.