ASTM D3242-11(2017)
(Test Method)Standard Test Method for Acidity in Aviation Turbine Fuel
Standard Test Method for Acidity in Aviation Turbine Fuel
SIGNIFICANCE AND USE
5.1 Some acids can be present in aviation turbine fuels due either to the acid treatment during the refining process or to naturally occurring organic acids. Significant acid contamination is not likely to be present because of the many check tests made during the various stages of refining. However, trace amounts of acid can be present and are undesirable because of the consequent tendencies of the fuel to corrode metals that it contacts or to impair the water separation characteristics of the aviation turbine fuel.
5.2 This test method is designed to measure the levels of acidity that can be present in aviation turbine fuel and is not suitable for determining significant acid contamination.
SCOPE
1.1 This test method covers the determination of the acidity in aviation turbine fuel in the range from 0.000 mg/g to 0.100 mg/g KOH.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3242 − 11 (Reapproved 2017)
Designation: 354/98
Standard Test Method for
Acidity in Aviation Turbine Fuel
This standard is issued under the fixed designation D3242; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope sample, required to titrate a sample in a specified solvent to a
specified endpoint using a specified detection system.
1.1 This test method covers the determination of the acidity
3.1.1.1 Discussion—in this test method, the solvent is a
in aviation turbine fuel in the range from 0.000 mg⁄g to
toluene-water-isopropanol mixture and the end point is deter-
0.100 mg⁄g KOH.
mined when a green/green brown color is obtained using the
1.2 The values stated in SI units are to be regarded as
specified p-naphtholbenzein indicator solution.
standard. No other units of measurement are included in this
4. Summary of Test Method
standard.
1.3 This standard does not purport to address all of the
4.1 The sample is dissolved in a mixture of toluene and
safety concerns, if any, associated with its use. It is the isopropyl alcohol containing a small amount of water. The
responsibility of the user of this standard to establish appro-
resulting single phase solution is blanketed by a stream of
priate safety and health practices and determine the applica- nitrogen bubbling through it and is titrated with standard
bility of regulatory limitations prior to use.
alcoholicpotassiumhydroxidetotheendpointindicatedbythe
1.4 This international standard was developed in accor- color change (orange in acid and green in base) of the added
dance with internationally recognized principles on standard-
p-naphtholbenzein solution.
ization established in the Decision on Principles for the
5. Significance and Use
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical 5.1 Some acids can be present in aviation turbine fuels due
Barriers to Trade (TBT) Committee. either to the acid treatment during the refining process or to
naturally occurring organic acids. Significant acid contamina-
2. Referenced Documents
tion is not likely to be present because of the many check tests
made during the various stages of refining. However, trace
2.1 ASTM Standards:
amounts of acid can be present and are undesirable because of
D664 Test Method for Acid Number of Petroleum Products
by Potentiometric Titration the consequent tendencies of the fuel to corrode metals that it
contacts or to impair the water separation characteristics of the
D1193 Specification for Reagent Water
aviation turbine fuel.
3. Terminology
5.2 This test method is designed to measure the levels of
3.1 Definitions:
acidity that can be present in aviation turbine fuel and is not
3.1.1 acid number, n—the quantity of a specified base,
suitable for determining significant acid contamination.
expressed in milligrams of potassium hydroxide per gram of
6. Apparatus
6.1 Buret—A 25 mL buret graduated in 0.1 mL
This test method is under the jurisdiction of ASTM Committee D02 on
subdivisions, or a 10 mL buret graduated in 0.05 mL subdivi-
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
Subcommittee D02.06 on Analysis of Liquid Fuels and Lubricants.
sions.
Current edition approved May 1, 2017. Published June 2017. Originally
approved in 1973. Last previous edition approved in 2011 as D3242 – 11. DOI: NOTE 1—An automated buret capable of delivering titrant amounts in
10.1520/D3242-11R17. 0.05 mL or smaller increments can be used, but the stated precision data
This test method has been approved by the sponsoring committees and accepted
were obtained using manual burets only.
by the cooperating societies in accordance with established procedures.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 7. Reagents and Materials
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
7.1 Purity of Reagents—Reagent grade chemicals shall be
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. used in all tests. Unless otherwise indicated, it is intended that
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3242 − 11 (2017)
all reagents shall conform to the specifications of the Commit-
tee onAnalytical Reagents of theAmerican Chemical Society,
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
NOTE 2—Commercially available reagents may be used in place of
laboratory preparations when they are certified in accordance with 7.1.
7.2 Purity of Water— References to water shall be under-
stood to mean distilled water as defined by Type III water of
Specification D1193.
4,5
7.3 p-Naphtholbenzein Indicator Solution—The
p-naphtholbenzeinmustmeetthespecificationsgiveninAnnex
A1.Prepareasolutionof p-naphtholbenzeinintitrationsolvent
equal to 10 g⁄L 6 0.01 g⁄L.
7.4 Nitrogen, dry-type, carbon dioxide-free. (Warning—
FIG. 1 Titration Flask
Compressed gas under high pressure. Gas reduces oxygen
available for breathing.)
7.5 Potassium Hydroxide Solution, Standard Alcoholic
(0.01 N)—Add 0.6 g of solid KOH (Warning—Highly corro-
the potassium hydroxide alcoholic solution to either of the
sive to all body tissue both in solid form and in solution.) to
following end points: (1) when the titration is electrometric,
approximately 1 L of anhydrous isopropyl alcohol
titrate to a well-defined inflection point at the voltage that
(Warning—Flammable.Vapor harmful. Keep away from heat,
corresponds to the voltage of the basic buffer solution; (2)
sparks, and open flame.) (containing less than 0.9 % water) in
when the titration is colorimetric, add 6 drops of phenolphtha-
a 2 L Erlenmeyer flask. Boil the mixture gently for 10 min to
lein indicator solution and titrate to the appearance of a
15 min, stirring to prevent the solids from forming a cake on
permanent pink color. Perform the blank titration on the water
the bottom. Add at least 0.2 g of barium hydroxide (Ba(OH) )
used to dissolve the potassium acid phthalate. Calculate the
(Warning—Poisonous if ingested. Strongly alkaline, causes
normality using the equation:
severe irritation producing dermatitis.) and again boil gently
W 1000
p
for 5 min to 10 min. Cool to room temperature, allow to stand
Normality 5 3 (1)
204.23 V 2 V
b
for several hours, and filter the supernatant liquid through a
fine sintered-glass or porcelain filtering funnel; avoid unnec-
where:
essary exposure to carbon dioxide (CO ) during filtration.
W = weight of the potassium acid phthalate, g,
p
Store the solution in a chemically resistant dispensing bottle
204.23 = molecular weight of the potassium acid phthalate,
out of contact with cork, rubber, or saponifiable stopcock
V = volume of titrant used to titrate the salt to the
lubricant and protected by a guard tube containing soda lime.
specific end point, mL, and
V = volume of titrant used to titrate the blank, mL.
NOTE 3—Because of the relative large coefficient of cubic expansion of b
organic liquids, such as isopropyl alcohol, the standard alcoholic solutions
7.5.2 Phenolphthalein Indicator Solution—Dissolve 0.1 g
should be standardized at temperatures close to those employed in the
6 0.01 g of pure solid phenolphthalein in 50 mLof water, free
titration of samples.
of CO , and 50 mL of ethanol.
7.5.1 Standardization of Potassium Hydroxide Solution—
7.6 Titration Solvent—Add 500 mL of toluene (Warning—
Standardize frequently enough to detect changes of 0.0002N.
Flammable. Vapor harmful. Keep away from heat, sparks, and
One way to accomplish this is as follows.Weigh, to the nearest
open flame.) and 5 mL of water to 495 mL of anhydrous
0.1 mg, approximately 0.02 g of potassium acid phthalate,
isopropyl alcohol.
which has been dried for at least 1 h at 110 °C 6 1 °C and
dissolve in 40 mL 6 1 mL of water, free of CO . Titrate with
8. Procedure
8.1 Introduce 100 g 6 5 g of the sample weighed to the
Reagent Chemicals, American Chemical Society Specifications, American
nearest 0.5 g, into a 500 mL wide-mouth Erlenmeyer flask.
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
listed by the American Chemical Society, see Annual Standards for Laboratory
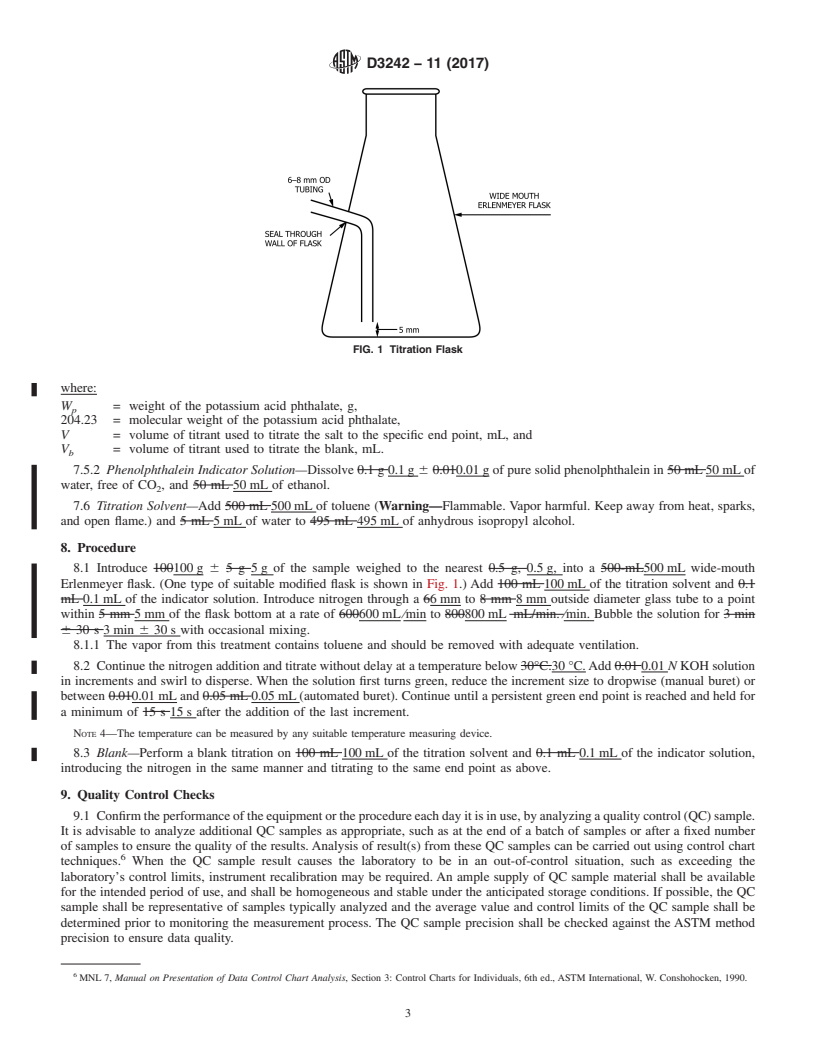
(One type of suitable modified flask is shown in Fig. 1.) Add
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
100 mL of the titration solvent and 0.1 mL of the indicator
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
solution. Introduce nitrogen through a 6 mm to 8 mm outside
MD.
diameter glass tube to a point within 5 mm of the flask bottom
In a 2006 study, only Kodak, Baker (Mallinkrodt), Fluka, and Aldrich were
found to meet the specifications in Annex A1. However, Kodak brand is no longer
at a rate of 600 mL⁄min to 800 mL⁄min. Bubble the solution
available.
for 3 min 6 30 s with occasional mixing.
Supporting data have been filed at ASTM International Headquarters and may
8.1.1 The vapor from this treatment contains toluene and
beobtainedbyrequestingResearchReportRR:D02-1626.ContactASTMCustomer
Service at service@astm.org. should be removed with adequate ventilation.
D3242 − 11 (2017)
A
TABLE 1 Precision
8.2 Continue the nitrogen addition and titrate without delay
at a temperature below 30 °C. Add 0.01 N KOH solution in
NOTE 1—All values are in acid number units.
increments and swirl to disperse. When the solution first turns
Average Acid Number Repeatability Reproducibility
green, reduce the increment size to dropwise (manual buret) or
0.001 0.0004 0.0013
between 0.01 mL and 0.05 mL (automated buret). Continue 0.002 0.0006 0.0018
0.005 0.0009 0.0029
until a persistent green end point is reached and held for a
0.010 0.0013 0.0041
minimum of 15 s after the addition of the last increment.
0.020 0.0019 0.0057
0.050 0.0030 0.0091
NOTE 4—The temperature can be measured by any suitable temperature
0.100 0.0042 0.0128
measuring device.
A
These precision data were derived as follows:
8.3 Blank—Perform a blank titration on 100 mL of the
Repeatability50.0132 a
œ
titration solvent and 0.1 mLof the indicator solution, introduc- Reproducibility50.0406 a
œ
where: a = acid number
ing the nitrogen in the same manner and titrating to the same
end point as above.
N = normality of the KOH solution, and
9. Quality Control Checks
W = sample used, g.
9.1 Confirm the performance of the equipment or the
11. Report
procedure each day it is in use, by analyzing a quality control
(QC) sample. It is advisable to analyze additional QC samples
11.1 Report the result to the nearest 0.001 mg KOH⁄g as
as appropriate, such as at the end of a batch of samples or after
Acid Number (Test Method D3242) = (Result).
a fixed number of samples to ensure the quality of the results.
12. Precision and Bias
Analysis of result(s) from these QC samples can be carried out
using control chart techniques. When the QC sample result 12.1 Precision—The precision of this test method as deter-
causes the laboratory to be in an out-of-control situation, such
mined by statistical examination of interlaboratory results is as
as exceeding the laboratory’s control limits, instrument recali- follows:
bration may be required. An ample supply of QC sample
12.1.1 Repeatability—The difference between two test
material shall be available for the intended period of use, and results, obtained by the same operator with the same apparatus
shall be homogeneous and stable under the anticipated storage under constant operating conditions on identical test material,
conditions. If possible, the QC sample shall be representative would in the long run, in the normal and correct operation of
of samples typically analyzed and the average value and the test method, exceed the following values only in one case
control limits of the QC sample shall be determined prior to in twenty (see Table 1).
monitoring the measurement process. The QC sample preci- 12.1.2 Reproducibility—The difference between two single
sion shall be checked against the ASTM method precision to and independent results obtained by different operators work-
ensure data quality. ing in different laboratories on identical test material would, in
the long run, in the normal and correct operation of the test
NOTE 5—Because the acid number can vary while the QC sample is in
method,exceedthefollowingvaluesonlyinonecaseintwenty
storage, when an out-of-control situation arises, the stability of the QC
(see Table 1).
sample can be a source of the error.
NOTE 6—The precision statements were based on the use of manual
10. Calculations
burets only. The user is cautioned that the precision statements may or
10.1 Calculate the acid number as follows: may not be applicable to titrations performed with the use of automated
burets, since no interlaboratory study has been conducted to date to
Acid number, mg of KOH/g 5 @~A 2 B!N 356.1#/W (2)
statistically evaluate results determined by both techniques.
where:
12.2 Bias—The procedure in this test method has no bias
because the value of the acid can be defined only in terms of
A = KOH solution required for titration of the sample (8.2),
the test method.
mL,
B = KOH solution required for titration of the blank (8.3),
13. Keywords
mL,
13.1 acidity; aviation turbine fuel
6 7
MNL 7, Manual on Presentation of Data Control Chart Analysis, Section 3: Supporting data have been filed at ASTM International Headquarters and may
Control Charts for Individuals, 6th ed., ASTM International, W. Conshohocken, beobtainedbyrequestingResearchReportRR:D02-1010.ContactASTMCustomer
1990. Service at service@astm.org.
D3242 − 11 (2017)
ANNEXES
(Mandatory Information)
A1. SPECIFICATIONS FOR p-NAPHTHOLBENZEIN
A1.1 Conformity Requirements A1.1.5 pH Range:
A1.1.5.1 Indicator turns to the first clear green at a relative
A1.1.1 Appearance—Red amorphous powder.
pH of 11 6 0.5 when tested by the method for pHr range of
A1.1.2 Chlorides—Less than 0.5 %. p-naphtholbenzein indicator as described in Annex
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3242 − 11 (Reapproved 2017)
Designation: 354/98
Standard Test Method for
Acidity in Aviation Turbine Fuel
This standard is issued under the fixed designation D3242; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope sample, required to titrate a sample in a specified solvent to a
specified endpoint using a specified detection system.
1.1 This test method covers the determination of the acidity
3.1.1.1 Discussion—in this test method, the solvent is a
in aviation turbine fuel in the range from 0.000 mg ⁄g to
toluene-water-isopropanol mixture and the end point is deter-
0.100 mg ⁄g KOH.
mined when a green/green brown color is obtained using the
1.2 The values stated in SI units are to be regarded as
specified p-naphtholbenzein indicator solution.
standard. No other units of measurement are included in this
standard. 4. Summary of Test Method
1.3 This standard does not purport to address all of the 4.1 The sample is dissolved in a mixture of toluene and
safety concerns, if any, associated with its use. It is the
isopropyl alcohol containing a small amount of water. The
responsibility of the user of this standard to establish appro- resulting single phase solution is blanketed by a stream of
priate safety and health practices and determine the applica-
nitrogen bubbling through it and is titrated with standard
bility of regulatory limitations prior to use. alcoholic potassium hydroxide to the end point indicated by the
1.4 This international standard was developed in accor-
color change (orange in acid and green in base) of the added
dance with internationally recognized principles on standard- p-naphtholbenzein solution.
ization established in the Decision on Principles for the
5. Significance and Use
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical 5.1 Some acids can be present in aviation turbine fuels due
Barriers to Trade (TBT) Committee. either to the acid treatment during the refining process or to
naturally occurring organic acids. Significant acid contamina-
2. Referenced Documents
tion is not likely to be present because of the many check tests
made during the various stages of refining. However, trace
2.1 ASTM Standards:
amounts of acid can be present and are undesirable because of
D664 Test Method for Acid Number of Petroleum Products
the consequent tendencies of the fuel to corrode metals that it
by Potentiometric Titration
D1193 Specification for Reagent Water contacts or to impair the water separation characteristics of the
aviation turbine fuel.
3. Terminology
5.2 This test method is designed to measure the levels of
3.1 Definitions:
acidity that can be present in aviation turbine fuel and is not
3.1.1 acid number, n—the quantity of a specified base,
suitable for determining significant acid contamination.
expressed in milligrams of potassium hydroxide per gram of
6. Apparatus
6.1 Buret—A 25 mL buret graduated in 0.1 mL
This test method is under the jurisdiction of ASTM Committee D02 on
subdivisions, or a 10 mL buret graduated in 0.05 mL subdivi-
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
Subcommittee D02.06 on Analysis of Liquid Fuels and Lubricants.
sions.
Current edition approved May 1, 2017. Published June 2017. Originally
NOTE 1—An automated buret capable of delivering titrant amounts in
approved in 1973. Last previous edition approved in 2011 as D3242 – 11. DOI:
10.1520/D3242-11R17. 0.05 mL or smaller increments can be used, but the stated precision data
This test method has been approved by the sponsoring committees and accepted were obtained using manual burets only.
by the cooperating societies in accordance with established procedures.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 7. Reagents and Materials
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
7.1 Purity of Reagents—Reagent grade chemicals shall be
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. used in all tests. Unless otherwise indicated, it is intended that
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3242 − 11 (2017)
all reagents shall conform to the specifications of the Commit-
tee on Analytical Reagents of the American Chemical Society,
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
NOTE 2—Commercially available reagents may be used in place of
laboratory preparations when they are certified in accordance with 7.1.
7.2 Purity of Water— References to water shall be under-
stood to mean distilled water as defined by Type III water of
Specification D1193.
4,5
7.3 p-Naphtholbenzein Indicator Solution—The
p-naphtholbenzein must meet the specifications given in Annex
A1. Prepare a solution of p-naphtholbenzein in titration solvent
equal to 10 g ⁄L 6 0.01 g ⁄L.
7.4 Nitrogen, dry-type, carbon dioxide-free. (Warning—
FIG. 1 Titration Flask
Compressed gas under high pressure. Gas reduces oxygen
available for breathing.)
7.5 Potassium Hydroxide Solution, Standard Alcoholic
(0.01 N)—Add 0.6 g of solid KOH (Warning—Highly corro-
the potassium hydroxide alcoholic solution to either of the
sive to all body tissue both in solid form and in solution.) to
following end points: (1) when the titration is electrometric,
approximately 1 L of anhydrous isopropyl alcohol
titrate to a well-defined inflection point at the voltage that
(Warning—Flammable. Vapor harmful. Keep away from heat,
corresponds to the voltage of the basic buffer solution; (2)
sparks, and open flame.) (containing less than 0.9 % water) in
when the titration is colorimetric, add 6 drops of phenolphtha-
a 2 L Erlenmeyer flask. Boil the mixture gently for 10 min to
lein indicator solution and titrate to the appearance of a
15 min, stirring to prevent the solids from forming a cake on
permanent pink color. Perform the blank titration on the water
the bottom. Add at least 0.2 g of barium hydroxide (Ba(OH) )
used to dissolve the potassium acid phthalate. Calculate the
(Warning—Poisonous if ingested. Strongly alkaline, causes
normality using the equation:
severe irritation producing dermatitis.) and again boil gently
W 1000
p
for 5 min to 10 min. Cool to room temperature, allow to stand
Normality5 3 (1)
204.23 V 2 V
b
for several hours, and filter the supernatant liquid through a
fine sintered-glass or porcelain filtering funnel; avoid unnec-
where:
essary exposure to carbon dioxide (CO ) during filtration.
W = weight of the potassium acid phthalate, g,
p
Store the solution in a chemically resistant dispensing bottle
204.23 = molecular weight of the potassium acid phthalate,
out of contact with cork, rubber, or saponifiable stopcock
V = volume of titrant used to titrate the salt to the
lubricant and protected by a guard tube containing soda lime.
specific end point, mL, and
V = volume of titrant used to titrate the blank, mL.
b
NOTE 3—Because of the relative large coefficient of cubic expansion of
organic liquids, such as isopropyl alcohol, the standard alcoholic solutions
7.5.2 Phenolphthalein Indicator Solution—Dissolve 0.1 g
should be standardized at temperatures close to those employed in the
6 0.01 g of pure solid phenolphthalein in 50 mL of water, free
titration of samples.
of CO , and 50 mL of ethanol.
7.5.1 Standardization of Potassium Hydroxide Solution—
7.6 Titration Solvent—Add 500 mL of toluene (Warning—
Standardize frequently enough to detect changes of 0.0002N.
Flammable. Vapor harmful. Keep away from heat, sparks, and
One way to accomplish this is as follows. Weigh, to the nearest
open flame.) and 5 mL of water to 495 mL of anhydrous
0.1 mg, approximately 0.02 g of potassium acid phthalate,
isopropyl alcohol.
which has been dried for at least 1 h at 110 °C 6 1 °C and
dissolve in 40 mL 6 1 mL of water, free of CO . Titrate with
8. Procedure
8.1 Introduce 100 g 6 5 g of the sample weighed to the
Reagent Chemicals, American Chemical Society Specifications, American
nearest 0.5 g, into a 500 mL wide-mouth Erlenmeyer flask.
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
listed by the American Chemical Society, see Annual Standards for Laboratory (One type of suitable modified flask is shown in Fig. 1.) Add
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
100 mL of the titration solvent and 0.1 mL of the indicator
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
solution. Introduce nitrogen through a 6 mm to 8 mm outside
MD.
diameter glass tube to a point within 5 mm of the flask bottom
In a 2006 study, only Kodak, Baker (Mallinkrodt), Fluka, and Aldrich were
found to meet the specifications in Annex A1. However, Kodak brand is no longer
at a rate of 600 mL ⁄min to 800 mL ⁄min. Bubble the solution
available.
for 3 min 6 30 s with occasional mixing.
Supporting data have been filed at ASTM International Headquarters and may
8.1.1 The vapor from this treatment contains toluene and
be obtained by requesting Research Report RR:D02-1626. Contact ASTM Customer
Service at service@astm.org. should be removed with adequate ventilation.
D3242 − 11 (2017)
A
TABLE 1 Precision
8.2 Continue the nitrogen addition and titrate without delay
at a temperature below 30 °C. Add 0.01 N KOH solution in
NOTE 1—All values are in acid number units.
increments and swirl to disperse. When the solution first turns
Average Acid Number Repeatability Reproducibility
green, reduce the increment size to dropwise (manual buret) or
0.001 0.0004 0.0013
0.002 0.0006 0.0018
between 0.01 mL and 0.05 mL (automated buret). Continue
0.005 0.0009 0.0029
until a persistent green end point is reached and held for a
0.010 0.0013 0.0041
minimum of 15 s after the addition of the last increment.
0.020 0.0019 0.0057
0.050 0.0030 0.0091
NOTE 4—The temperature can be measured by any suitable temperature
0.100 0.0042 0.0128
measuring device.
A
These precision data were derived as follows:
8.3 Blank—Perform a blank titration on 100 mL of the
Repeatability 50.0132 a
œ
Reproducibility 50.0406 a
titration solvent and 0.1 mL of the indicator solution, introduc-
œ
where: a = acid number
ing the nitrogen in the same manner and titrating to the same
end point as above.
N = normality of the KOH solution, and
9. Quality Control Checks
W = sample used, g.
9.1 Confirm the performance of the equipment or the
11. Report
procedure each day it is in use, by analyzing a quality control
(QC) sample. It is advisable to analyze additional QC samples
11.1 Report the result to the nearest 0.001 mg KOH ⁄g as
as appropriate, such as at the end of a batch of samples or after
Acid Number (Test Method D3242) = (Result).
a fixed number of samples to ensure the quality of the results.
12. Precision and Bias
Analysis of result(s) from these QC samples can be carried out
using control chart techniques. When the QC sample result
12.1 Precision—The precision of this test method as deter-
causes the laboratory to be in an out-of-control situation, such mined by statistical examination of interlaboratory results is as
as exceeding the laboratory’s control limits, instrument recali-
follows:
bration may be required. An ample supply of QC sample 12.1.1 Repeatability—The difference between two test
material shall be available for the intended period of use, and results, obtained by the same operator with the same apparatus
shall be homogeneous and stable under the anticipated storage under constant operating conditions on identical test material,
conditions. If possible, the QC sample shall be representative would in the long run, in the normal and correct operation of
of samples typically analyzed and the average value and the test method, exceed the following values only in one case
control limits of the QC sample shall be determined prior to in twenty (see Table 1).
monitoring the measurement process. The QC sample preci- 12.1.2 Reproducibility—The difference between two single
sion shall be checked against the ASTM method precision to and independent results obtained by different operators work-
ensure data quality. ing in different laboratories on identical test material would, in
the long run, in the normal and correct operation of the test
NOTE 5—Because the acid number can vary while the QC sample is in
method, exceed the following values only in one case in twenty
storage, when an out-of-control situation arises, the stability of the QC
(see Table 1).
sample can be a source of the error.
NOTE 6—The precision statements were based on the use of manual
10. Calculations
burets only. The user is cautioned that the precision statements may or
10.1 Calculate the acid number as follows: may not be applicable to titrations performed with the use of automated
burets, since no interlaboratory study has been conducted to date to
Acid number, mg of KOH/g 5 A 2 B N 3 56.1 /W (2)
@~ ! #
statistically evaluate results determined by both techniques.
where:
12.2 Bias—The procedure in this test method has no bias
because the value of the acid can be defined only in terms of
A = KOH solution required for titration of the sample (8.2),
the test method.
mL,
B = KOH solution required for titration of the blank (8.3),
13. Keywords
mL,
13.1 acidity; aviation turbine fuel
6 7
MNL 7, Manual on Presentation of Data Control Chart Analysis, Section 3: Supporting data have been filed at ASTM International Headquarters and may
Control Charts for Individuals, 6th ed., ASTM International, W. Conshohocken, be obtained by requesting Research Report RR:D02-1010. Contact ASTM Customer
1990. Service at service@astm.org.
D3242 − 11 (2017)
ANNEXES
(Mandatory Information)
A1. SPECIFICATIONS FOR p-NAPHTHOLBENZEIN
A1.1 Conformity Requirements A1.1.5 pH Range:
A1.1.5.1 Indicator turns to the first clear green at a relative
A1.1.1 Appearance—Red amorphous powder.
pH of 11 6 0.5 when tested by the method for pHr range of
A1.1.2 Chlorides—Less than 0.5 %.
p-naphtholbenzein indicator as described in Annex A2.
A1.1.5.2 Requires not more than 0.5 m
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D3242 − 11 D3242 − 11 (Reapproved 2017)
Designation: 354/98
Standard Test Method for
Acidity in Aviation Turbine Fuel
This standard is issued under the fixed designation D3242; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope*Scope
1.1 This test method covers the determination of the acidity in aviation turbine fuel in the range from 0.0000.000 mg ⁄g to
0.1000.100 mg mg KOH/g.⁄g KOH.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D664 Test Method for Acid Number of Petroleum Products by Potentiometric Titration
D1193 Specification for Reagent Water
3. Terminology
3.1 Definitions:
3.1.1 acid number, n—the quantity of a specified base, expressed in milligrams of potassium hydroxide per gram of sample,
required to titrate a sample in a specified solvent to a specified endpoint using a specified detection system.
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcommittee
D02.06 on Analysis of Liquid Fuels and Lubricants.
Current edition approved May 1, 2011May 1, 2017. Published July 2011June 2017. Originally approved in 1973. Last previous edition approved in 20082011 as
D3242D3242 – 11.–08. DOI: 10.1520/D3242-11.10.1520/D3242-11R17.
This test method has been approved by the sponsoring committees and accepted by the cooperating societies in accordance with established procedures.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3.1.1.1 Discussion—
in this test method, the solvent is a toluene-water-isopropanol mixture and the end point is determined when a green/green brown
color is obtained using the specified p-naphtholbenzein indicator solution.
4. Summary of Test Method
4.1 The sample is dissolved in a mixture of toluene and isopropyl alcohol containing a small amount of water. The resulting
single phase solution is blanketed by a stream of nitrogen bubbling through it and is titrated with standard alcoholic potassium
hydroxide to the end point indicated by the color change (orange in acid and green in base) of the added p-naphtholbenzein
solution.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3242 − 11 (2017)
5. Significance and Use
5.1 Some acids can be present in aviation turbine fuels due either to the acid treatment during the refining process or to naturally
occurring organic acids. Significant acid contamination is not likely to be present because of the many check tests made during
the various stages of refining. However, trace amounts of acid can be present and are undesirable because of the consequent
tendencies of the fuel to corrode metals that it contacts or to impair the water separation characteristics of the aviation turbine fuel.
5.2 This test method is designed to measure the levels of acidity that can be present in aviation turbine fuel and is not suitable
for determining significant acid contamination.
6. Apparatus
6.1 Buret—A 25-mL25 mL buret graduated in 0.1-mL0.1 mL subdivisions, or a 10-mL10 mL buret graduated in 0.05-
mL0.05 mL subdivisions.
NOTE 1—An automated buret capable of delivering titrant amounts in 0.05 mL 0.05 mL or smaller increments can be used, but the stated precision
data were obtained using manual burets only.
7. Reagents and Materials
7.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
NOTE 2—Commercially available reagents may be used in place of laboratory preparations when they are certified in accordance with 7.1.
7.2 Purity of Water— References to water shall be understood to mean distilled water as defined by Type III water of
Specification D1193.
4,5
7.3 p-Naphtholbenzein Indicator Solution—The p-naphtholbenzein must meet the specifications given in Annex A1. Prepare
a solution of p-naphtholbenzein in titration solvent equal to 1010 g ⁄L 6 0.01 0.01 g g/L.⁄L.
7.4 Nitrogen, dry-type, carbon dioxide-free. (Warning—Compressed gas under high pressure. Gas reduces oxygen available for
breathing.)
7.5 Potassium Hydroxide Solution, Standard Alcoholic (0.01 N)—Add 0.6 g 0.6 g of solid KOH (Warning—Highly corrosive
to all body tissue both in solid form and in solution.) to approximately 1 L 1 L of anhydrous isopropyl alcohol (Warning—
Flammable. Vapor harmful. Keep away from heat, sparks, and open flame.) (containing less than 0.9 % water) in a 2-L2 L
Erlenmeyer flask. Boil the mixture gently for 1010 min to 15 min, 15 min, stirring to prevent the solids from forming a cake on
the bottom. Add at least 0.2 g 0.2 g of barium hydroxide (Ba(OH) ) (Warning—Poisonous if ingested. Strongly alkaline, causes
severe irritation producing dermatitis.) and again boil gently for 55 min to 10 min. 10 min. Cool to room temperature, allow to
stand for several hours, and filter the supernatant liquid through a fine sintered-glass or porcelain filtering funnel; avoid
unnecessary exposure to carbon dioxide (CO ) during filtration. Store the solution in a chemically resistant dispensing bottle out
of contact with cork, rubber, or saponifiable stopcock lubricant and protected by a guard tube containing soda lime.
NOTE 3—Because of the relative large coefficient of cubic expansion of organic liquids, such as isopropyl alcohol, the standard alcoholic solutions
should be standardized at temperatures close to those employed in the titration of samples.
7.5.1 Standardization of Potassium Hydroxide Solution—Standardize frequently enough to detect changes of 0.0002N. One way
to accomplish this is as follows. Weigh, to the nearest 0.1 mg, approximately 0.02 g 0.1 mg, approximately 0.02 g of potassium
acid phthalate, which has been dried for at least 1 h 1 h at 110110 °C 6 1°C1 °C and dissolve in 4040 mL 6 1 mL 1 mL of water,
free of CO . Titrate with the potassium hydroxide alcoholic solution to either of the following end points: (1) when the titration
is electrometric, titrate to a well-defined inflection point at the voltage that corresponds to the voltage of the basic buffer solution;
(2) when the titration is colorimetric, add 6 drops of phenolphthalein indicator solution and titrate to the appearance of a permanent
pink color. Perform the blank titration on the water used to dissolve the potassium acid phthalate. Calculate the normality using
the equation:
W 1000
p
Normality 5 3 (1)
204.23 V 2 V
b
where:
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For Suggestions on the testing of reagents not listed by
the American Chemical Society, see Annual Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
In a 2006 study, only Kodak, Baker (Mallinkrodt), Fluka, and Aldrich were found to meet the specifications in Annex A1. However, Kodak brand is no longer available.
Supporting data have been filed at ASTM International Headquarters and may be obtained by requesting Research Report RR:D02-1626. Contact ASTM Customer
Service at service@astm.org.
D3242 − 11 (2017)
FIG. 1 Titration Flask
where:
W = weight of the potassium acid phthalate, g,
p
204.23 = molecular weight of the potassium acid phthalate,
V = volume of titrant used to titrate the salt to the specific end point, mL, and
V = volume of titrant used to titrate the blank, mL.
b
7.5.2 Phenolphthalein Indicator Solution—Dissolve 0.1 g 0.1 g 6 0.010.01 g of pure solid phenolphthalein in 50 mL 50 mL of
water, free of CO , and 50 mL 50 mL of ethanol.
7.6 Titration Solvent—Add 500 mL 500 mL of toluene (Warning—Flammable. Vapor harmful. Keep away from heat, sparks,
and open flame.) and 5 mL 5 mL of water to 495 mL 495 mL of anhydrous isopropyl alcohol.
8. Procedure
8.1 Introduce 100100 g 6 5 g 5 g of the sample weighed to the nearest 0.5 g, 0.5 g, into a 500-mL500 mL wide-mouth
Erlenmeyer flask. (One type of suitable modified flask is shown in Fig. 1.) Add 100 mL 100 mL of the titration solvent and 0.1
mL 0.1 mL of the indicator solution. Introduce nitrogen through a 66 mm to 8 mm 8 mm outside diameter glass tube to a point
within 5 mm 5 mm of the flask bottom at a rate of 600600 mL ⁄min to 800800 mL mL/min. ⁄min. Bubble the solution for 3 min
6 30 s 3 min 6 30 s with occasional mixing.
8.1.1 The vapor from this treatment contains toluene and should be removed with adequate ventilation.
8.2 Continue the nitrogen addition and titrate without delay at a temperature below 30°C.30 °C. Add 0.01 0.01 N KOH solution
in increments and swirl to disperse. When the solution first turns green, reduce the increment size to dropwise (manual buret) or
between 0.010.01 mL and 0.05 mL 0.05 mL (automated buret). Continue until a persistent green end point is reached and held for
a minimum of 15 s 15 s after the addition of the last increment.
NOTE 4—The temperature can be measured by any suitable temperature measuring device.
8.3 Blank—Perform a blank titration on 100 mL 100 mL of the titration solvent and 0.1 mL 0.1 mL of the indicator solution,
introducing the nitrogen in the same manner and titrating to the same end point as above.
9. Quality Control Checks
9.1 Confirm the performance of the equipment or the procedure each day it is in use, by analyzing a quality control (QC) sample.
It is advisable to analyze additional QC samples as appropriate, such as at the end of a batch of samples or after a fixed number
of samples to ensure the quality of the results. Analysis of result(s) from these QC samples can be carried out using control chart
techniques. When the QC sample result causes the laboratory to be in an out-of-control situation, such as exceeding the
laboratory’s control limits, instrument recalibration may be required. An ample supply of QC sample material shall be available
for the intended period of use, and shall be homogeneous and stable under the anticipated storage conditions. If possible, the QC
sample shall be representative of samples typically analyzed and the average value and control limits of the QC sample shall be
determined prior to monitoring the measurement process. The QC sample precision shall be checked against the ASTM method
precision to ensure data quality.
MNL 7, Manual on Presentation of Data Control Chart Analysis, Section 3: Control Charts for Individuals, 6th ed., ASTM International, W. Conshohocken, 1990.
D3242 − 11 (2017)
A
TABLE 1 Precision
NOTE 1—All values are in acid number units.
Average Acid Number Repeatability Reproducibility
0.001 0.0004 0.0013
0.002 0.0006 0.0018
0.005 0.0009 0.0029
0.010 0.0013 0.0041
0.020 0.0019 0.0057
0.050 0.0030 0.0091
0.100 0.0042 0.0128
A
These precision data were derived as follows:
Repeatability 50.0132 a
œ
Reproducibility 50.0406 a
œ
where: a = acid number
NOTE 5—Because the acid number can vary while the QC sample is in storage, when an out-of-control situation arises, the stability of the QC sample
can be a source of the error.
10. Calculations
10.1 Calculate the acid number as follows:
Acid number, mg of KOH/g5 @~A 2 B!N 356.1#/W (2)
where:
where:
A = KOH solution required for titration of the sample (8.2), mL,
B = KOH solution required for titration of the blank (8.3), mL,
N = normality of the KOH solution, and
W = sample used, g.
11. Report
11.1 Report the result to the nearest 0.001 mg0.001 mg KOH KOH/g ⁄g as Acid Number (Test Method D3242) = (Result).
12. Precision and Bias
12.1 Precision—The precision of this test method as determined by statistical examination of interlaboratory results is as
follows:
12.1.1 Repeatability—The difference between two test results, obtained by the same operator with the same apparatus under
constant operating conditions on identical test material, would in the long run, in the normal and correct operation of the test
method, exceed the following values only in one case in twenty (see Table 1).
12.1.2 Reproducibility—The difference between two single and independent results obtained by different operators working in
different laboratories on identical test material would, in the long run, in the normal and correct operation of the test method,
exceed the following values only in one case in twenty (see Table 1).
NOTE 6—The precision statements were based on the use of manual burets only. The user is cautioned that the precision statements may or may not
be applicable to titrations performed with the use of automated burets, since no interlaboratory study has been conducted to date to statistically evaluate
results determined by both techniques.
12.2 Bias—The procedure in this test method has no bias because the value of the acid can be defined only in terms of
...











Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.