ASTM D2878-95(2005)
(Test Method)Standard Test Method for Estimating Apparent Vapor Pressures and Molecular Weights of Lubricating Oils
Standard Test Method for Estimating Apparent Vapor Pressures and Molecular Weights of Lubricating Oils
SIGNIFICANCE AND USE
The vapor pressure of a substance as determined by measurement of evaporation reflects a property of the bulk sample. Little weight is given by the procedure to the presence of low concentrations of volatile impurities.
Vapor pressure, per se, is a thermodynamic property that is dependent only upon composition and temperature for stable systems. In the present method, composition changes occur during the course of the test so that the contribution of minor amounts of volatile impurities is minimized.
SCOPE
1.1 This test method provides a calculation procedure for converting data obtained by Test Method D 972 to apparent vapor pressures and molecular weights. It has been demonstrated to be applicable to petroleum-based and synthetic ester lubricating oils, at temperatures of 395 to 535K (250 to 500oF). However, its applicability to lubricating greases has not been established.
Note 1--Most lubricants boil over a fairly wide temperature range, a fact recognized in discussion of their vapor pressures. For example, the apparent vapor pressure over the range 0 to 0.1 % evaporated may be as much as 100 times that over the range 4.9 to 5.0 % evaporated.
1.2 The values stated in SI units are to be regarded as the standard. In cases in which materials, products, or equipment are available in inch-pound units only, SI units are omitted.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability or regulatory limitations prior to use. For specific hazard statements, see 6.2, 7.1, and 8.2.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2878–95 (Reapproved 2005)
Standard Test Method for
Estimating Apparent Vapor Pressures and Molecular
Weights of Lubricating Oils
This standard is issued under the fixed designation D2878; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D92 Test Method for Flash and Fire Points by Cleveland
Open Cup Tester
1.1 This test method provides a calculation procedure for
D972 Test Method for Evaporation Loss of Lubricating
converting data obtained by Test Method D972 to apparent
Greases and Oils
vapor pressures and molecular weights. It has been demon-
D2503 Test Method for Relative Molecular Mass (Molecu-
strated to be applicable to petroleum-based and synthetic ester
2 lar Weight) of Hydrocarbons by Thermoelectric Measure-
lubricating oils, at temperatures of 395 to 535K (250 to
ment of Vapor Pressure
500°F). However, its applicability to lubricating greases has
D2595 Test Method for Evaporation Loss of Lubricating
not been established.
Greases Over Wide-Temperature Range
NOTE 1—Most lubricants boil over a fairly wide temperature range, a
D2883 Test Method for ReactionThresholdTemperature of
fact recognized in discussion of their vapor pressures. For example, the
Liquid and Solid Materials
apparent vapor pressure over the range 0 to 0.1% evaporated may be as
E1 Specification for ASTM Liquid-in-Glass Thermometers
much as 100 times that over the range 4.9 to 5.0% evaporated.
E659 Test Method for Autoignition Temperature of Liquid
1.2 The values stated in SI units are to be regarded as the
Chemicals
standard. In cases in which materials, products, or equipment
are available in inch-pound units only, SI units are omitted.
3. Terminology
1.3 This standard does not purport to address all of the
3.1 Definitions of Terms Specific to This Standard:
safety concerns, if any, associated with its use. It is the
3.1.1 apparent vapor pressure (p), n—the time-averaged
responsibility of the user of this standard to establish appro-
value of the vapor pressure from the start to the end of the
priate safety and health practices and determine the applica-
evaporation test.
bility or regulatory limitations prior to use. For specific
3.1.1.1 Discussion—Whilethismayincludesomeeffectsof
warning statements, see 6.2, 7.1, 8.2, and Annex A2.
differences in nonideality of the vapor, heat of vaporization,
surface tension, and viscosity between the m-terphenyl and the
2. Referenced Documents
lubricating oil, these factors have been demonstrated to be
2.1 ASTM Standards:
negligible.Unlessstated,thisaverageshallcovertherange0to
A240/A240M Specification for Chromium and Chromium-
5 61%.
Nickel Stainless Steel Plate, Sheet, and Strip for Pressure
3.1.2 cell constant (k), n—the ratio of the amount of
Vessels and for General Applications
m-terphenylorlubricatingoilcarriedoffperunitvolumeofgas
to that predicted by Dalton’s law.
k 522.41 PW/VpM (1)
This test method is under the jurisdiction of Committee D02 on Petroleum
ProductsandLubricantsandisthedirectresponsibilityofSubcommitteeD02.11on
where:
Engineering Sciences of High Performance Fluids and Solids.
k = call constant
Current edition approved June 1, 2005. Published September 2005. Originally
´1
approved in 1970. Last previous edition approved in 2000 as D2878–95(2000) .
P = ambient atmospheric pressure, torr
DOI: 10.1520/D2878-95R05.
W = mass of lubricant evaporated, g
Coburn, J. F., “Lubricant Vapor Pressure Derived from Evaporation Loss,”
V = volume of gas passed through all litres at 273K and
Transactions,AmericanSocietyofLubricatingEngineers,ASLTA,Vol12,1969,pp.
101.3 kPa (760 torr)
129–134.
p = apparent vapor pressure, torr
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
M = mole average molecular weight of lubricant vapor,
Standards volume information, refer to the standard’s Document Summary page on
g/mole
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2878–95 (2005)
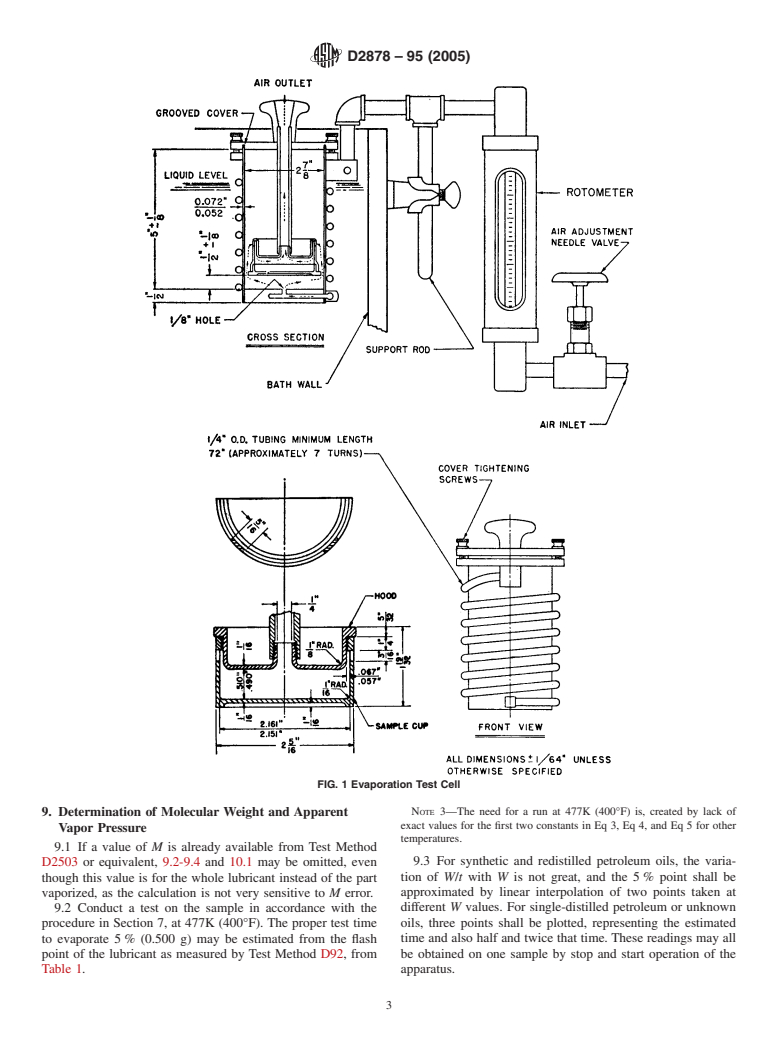
6.6 Oil Sample Cup, as described in Fig. 1 and A1.1.2.
T = test temperature, K
Ithasbeenempiricallydeterminedthatfor m-terphenylinair
7. Calibration of Equipment
k 50.1266 212.60/~ T 2273! (2)
7.1 ItisassumedthatequipmentconformingtoTestMethod
and that the cell constant is independent of the composition
D972 in design and installation needs no calibration. If
of the lubricant.
questions arise, carry out the procedure using m-terphenyl
3.1.3 Test Method D972 is normally run with air, which
(Warning—Harmful or fatal if swallowed. SeeA2.2.) of good
may cause changes in easily oxidized fluids. In such cases, use 5
commercial quality. The following two points shall be deter-
of common reactive gas nitrogen and recalibration to obtain a
mined:
slightly different cell constant (k8) is mandatory.
Temperature Evaporation to Conform
to Eq 2, g
K °F Time, h
4. Summary of Test Method
395 250 22 0.267 6 0.027
4.1 Thetestisrunattheselectedtemperatureforasufficient
420 300 6.5 0.503 6 0.050
time to give the selected amount of evaporation, which is 5 6
If the data do not fall within the above ranges, check flow
1% unless otherwise specified. This evaporation rate is com-
rate and temperature. If these are correct, prepare a substitute
pared with a standard value for pure m-terphenyl to yield the
equation for k8 similar to Eq 2 and use it in Section 10. When
apparent vapor pressure and molecular weight of the lubricat-
use of nonreactive gas is required, this calibration is necessary
ing oil as defined in Section 3.
as standard cell constants are not valid for gases other than air.
5. Significance and Use
7.2 If the apparatus specified inTest Method D2595 is to be
used, it shall be calibrated as described in 7.1.
5.1 The vapor pressure of a substance as determined by
measurement of evaporation reflects a property of the bulk
8. Procedure
sample. Little weight is given by the procedure to the presence
of low concentrations of volatile impurities. 8.1 Weigh the clean test specimen cup and hood to the
5.2 Vaporpressure, per se,isathermodynamicpropertythat nearest 1 mg.Transfer, by means of a pipet, 10.00 6 0.05 g of
isdependentonlyuponcompositionandtemperatureforstable test specimen to the cup. Assemble the cup and hood, being
systems. In the present method, composition changes occur careful not to splash oil on the underside of the hood. Weigh
during the course of the test so that the contribution of minor the assembly and record the net test specimen weight to the
amounts of volatile impurities is minimized. nearest 1 mg.
8.2 With cover in place, but without the hood and test
6. Apparatus
specimen cup attached, allow the evaporation cell to acquire
6.1 Evaporation Cell, as described in Annex A1. the temperature of the bath (controlled to 60.5K (61°F)) at
6.2 Air Supply System, capable of supplying to the cell the
which the test is to be made by immersing the cell in it, as
required flow of air free of entrained particles (Warning— shown in Fig. 1.Allow the cell to remain in the bath at least ⁄2
Compressedgasunderhighpressure.Usewithextremecaution
h before beginning the test. During this period, allow clean air
in the presence of combustible material, since the autoignition (Warning—Compressed gas under high pressure. Use with
temperatures of most organic compounds in air are drastically
extreme caution in the presence of combustible material, since
reduced at elevated pressures. See Annex A2.1.). A 410-mm theautoignitiontemperaturesofmostorganiccompoundsinair
(16-in.) length of 1-in. diameter pipe packed with glass wool
are drastically reduced at elevated pressures. SeeAnnexA2.1.)
has been found satisfactory for filtering the air. to flow through the cell at the prescribed rate, 2.583 6 0.02
6.3 Oil Bath, as described in Annex A1.
g/min (2 L/min at standard temperature and pressure), as
indicated by the rotameter. Then remove the cover, thread and
NOTE 2—Other constant-temperature baths may be used if the exit air
weighedhoodandsamplecupintoplace,andreplacethecover.
passing over the grease sample is at the test temperature (60.5K (1°F)).
Tighten the three knurled cover-tightening screws securely to
6.4 Thermometers—ASTM thermometers graduated in ei-
prevent air leakage under the cover. Pass clean air through the
ther Celsius or Fahrenheit degrees and having a range from−5
cell for the required period. (Warning—Do not perform this
to400°C(20to760°F)andconformingtotherequirementsfor
test with air at temperatures in excess of the autoignition
Thermometers 3C or 3F, respectively, as described in Specifi-
temperatureofthetestspecimenasdeterminedbyTestMethod
cation E1.
E659 or Test Method D2883, or both.)
6.5 Flowmeter —A rotameter calibrated to deliver air at a
8.3 At the end of the test period, remove the assembled test
rate of 2.583 6 0.02 g/min between 289 and 302K (60 and
specimen cup and hood from the cell, and allow to cool to
85°F) (2 L/min at standard temperature and pressure). It shall
room temperature. Determine the net weight of the sample to
befurnishedwithaneedlevalveandmountedasshowninFig.
the nearest 1 mg.
1.
4 5
The sole source of supply of the apparatus known to the committee at this time The sole source of supply of the apparatus known to the committee at this time
is Flowrater meter, Fisher and Porter Co., Hatboro, PA. If you are aware of is Santowax, M., Monsanto Chemical Co., St. Louis, MO. If you are aware of
alternative suppliers, please provide this information to ASTM International alternative suppliers, please provide this information to ASTM International
Headquarters.Your comments will receive careful consideration at a meeting of the Headquarters.Your comments will receive careful consideration at a meeting of the
responsible technical committee, which you may attend. responsible technical committee, which you may attend.
D2878–95 (2005)
FIG. 1 Evaporation Test Cell
NOTE 3—The need for a run at 477K (400°F) is, created by la
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.