ASTM D4763-06(2020)
(Practice)Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy
Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy
SIGNIFICANCE AND USE
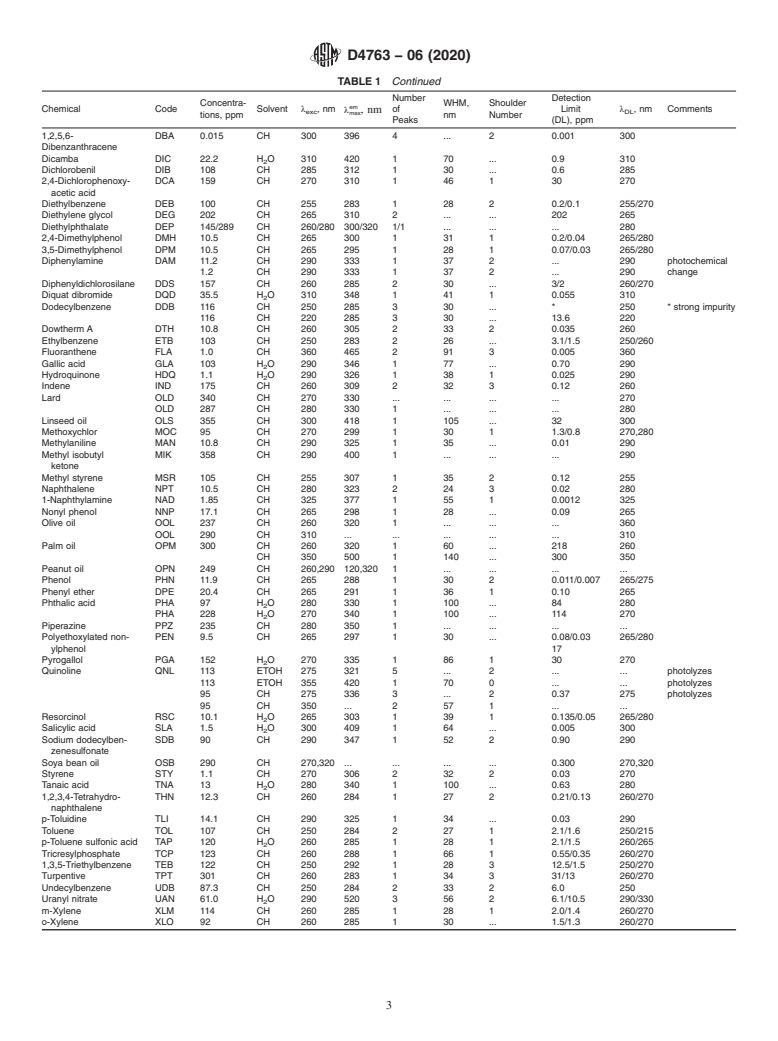
5.1 This practice is useful for detecting and identifying (or determining the absence of) 90 chemicals with relatively high fluorescence yields (see Table 1). Most commonly, this practice will be useful for distinguishing single fluorescent chemicals in solution, simple mixtures or single fluorescing chemicals in the presence of other nonfluorescing chemicals. Chemicals with high fluorescence yields tend to have aromatic rings, some heterocyclic rings or extended conjugated double-bond systems. Typical chemicals included on this list include aromatics, substituted aromatics such as phenols, polycyclic aromatic hydrocarbons (PAH’s), some pesticides such as DDT, polychlorinated biphenyls (PCB’s), some heterocyclics, and some esters, organic acids, and ketones.
5.2 With appropriate separatory techniques (HPLC, TLC, and column chromatography) and in some cases, special detection techniques (OMA’s and diode arrays), this practice can be used to determine these 90 chemicals even in complex mixtures containing a number of other fluorescing chemicals. With the use of appropriate excitation and emission wavelengths and prior generation of calibration curves, this practice could be used for quantitation of these chemicals over a broad linear range.
5.3 Fluorescence is appropriately a trace technique and at higher concentrations (greater than 10 to 100 ppm) spectral distortions usually due to self-absorption, or inner-filter effects but sometimes ascribed to fluorescence quenching, may be observed. These effects can usually be eliminated by diluting the solution. Detection limits can be lowered following identification by using broader slit widths, but this may result in spectral broadening and distortion.
5.4 This practice assumes the use of a corrected spectrofluorometer (that is, one capable of producing corrected fluorescence spectra). On an uncorrected instrument, peak shifts and spectral distortions and changes in peak ratios may be noted. An uncorrected spect...
SCOPE
1.1 This practice allows for the identification of 90 chemicals that may be found in water or in surface layers on water. This practice is based on the use of room-temperature fluorescence spectra taken from lists developed by the U.S. Environmental Protection Agency and the U.S. Coast Guard (1). Ref (1) is the primary source for these spectra. This practice is also based on the assumption that such chemicals are either present in aqueous solution or are extracted from water into an appropriate solvent.2
1.2 Although many organic chemicals containing aromatic rings, heterocyclic rings, or extended conjugated double-bond systems have appreciable quantum yields of fluorescence, this practice is designed only for the specific compounds listed. If present in complex mixtures, preseparation by high-performance liquid chromatography (HPLC), column chromatography, or thin-layer chromatography (TLC) would probably be required.
1.3 If used with HPLC, this practice could be used for the identification of fluorescence spectra generated by optical multichannel analyzers (OMA) or diode-array detectors.
1.4 For simple mixtures, or in the presence of other nonfluorescing chemicals, separatory techniques might not be required. The excitation and emission maximum wavelengths listed in this practice could be used with standard fluorescence techniques (see Refs (2-6)) to quantitate these ninety chemicals once identification had been established. For such uses, generation of a calibration curve, to determine the linear range for use of fluorescence quantitation would be required for each chemical. Examination of solvent blanks to subtract or eliminate any fluorescence background would probably be required.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practi...
General Information
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation:D4763 −06 (Reapproved 2020)
Standard Practice for
Identification of Chemicals in Water by Fluorescence
1
Spectroscopy
This standard is issued under the fixed designation D4763; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope responsibility of the user of this standard to establish appro-
priate safety, health, and environmental practices and deter-
1.1 This practice allows for the identification of 90 chemi-
mine the applicability of regulatory limitations prior to use.
cals that may be found in water or in surface layers on water.
1.6 This international standard was developed in accor-
This practice is based on the use of room-temperature fluores-
dance with internationally recognized principles on standard-
cence spectra taken from lists developed by the U.S. Environ-
ization established in the Decision on Principles for the
mental Protection Agency and the U.S. Coast Guard (1). Ref
Development of International Standards, Guides and Recom-
(1) is the primary source for these spectra. This practice is also
mendations issued by the World Trade Organization Technical
based on the assumption that such chemicals are either present
Barriers to Trade (TBT) Committee.
in aqueous solution or are extracted from water into an
2
appropriate solvent.
2. Referenced Documents
1.2 Although many organic chemicals containing aromatic
3
2.1 ASTM Standards:
rings, heterocyclic rings, or extended conjugated double-bond
D1129 Terminology Relating to Water
systems have appreciable quantum yields of fluorescence, this
D1193 Specification for Reagent Water
practice is designed only for the specific compounds listed. If
E131 Terminology Relating to Molecular Spectroscopy
present in complex mixtures, preseparation by high-
E275 Practice for Describing and Measuring Performance of
performance liquid chromatography (HPLC), column
Ultraviolet and Visible Spectrophotometers
chromatography, or thin-layer chromatography (TLC) would
probably be required.
3. Terminology
1.3 If used with HPLC, this practice could be used for the
3.1 Definitions—For definitions of terms used in this
identification of fluorescence spectra generated by optical
practice, refer to Terminology D1129, Specification D1193,
multichannel analyzers (OMA) or diode-array detectors.
and definitions under the jurisdiction of Committee E13 such
1.4 For simple mixtures, or in the presence of other non-
as Terminology E131 and Practice E275.
fluorescing chemicals, separatory techniques might not be
4. Summary of Practice
required. The excitation and emission maximum wavelengths
listed in this practice could be used with standard fluorescence
4.1 This practice uses well tested fluorescence techniques to
techniques(seeRefs (2-6))toquantitatetheseninetychemicals
detect and identify (or determine the absence of) 90 chemicals
once identification had been established. For such uses, gen-
that have relatively high fluorescence yields. Table 1 lists for
eration of a calibration curve, to determine the linear range for
each chemical an appropriate solvent (either cyclohexane,
use of fluorescence quantitation would be required for each
water, methyl or ethyl alcohol, depending on solubility), a
chemical. Examination of solvent blanks to subtract or elimi-
suggested excitation wavelength for maximum sensitivity, a
nate any fluorescence background would probably be required.
wavelength corresponding to the emission maximum, the
1.5 This standard does not purport to address all of the number of fluorescence peaks and shoulders, the width (full
safety concerns, if any, associated with its use. It is the width at half of the maximum emission intensity) of the
strongest fluorescence peak and the detection limit for the
experimental conditions given. Detection limits could be
1
This practice is under the jurisdiction of ASTM Committee D19 on Water and
lowered, following identification, by using broader slit widths.
is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
Organic Substances in Water.
Current edition approved Dec. 15, 2020. Published December 2020. Originally
3
approved in 1988. Last previous edition approved in 2012 as D4763 – 06 (2012). For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/D4763-06R20. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
2
The boldface numbers in parentheses refer to a list of references at the end of Sta
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.