ASTM D3263-82(1999)e1
(Test Method)Standard Test Methods for Corrosivity of Solvent Systems for Removing Water-Formed Deposits
Standard Test Methods for Corrosivity of Solvent Systems for Removing Water-Formed Deposits
SCOPE
1.1 These test methods cover the determination of corrosivity of solvent systems used to remove water-formed deposits from the metal and alloy surfaces of water handling equipment. Four test methods are given as follows: Sections Test Method A-Corrosivity in the Absence of Deposits 10 to 15 Test Method B-Corrosivity in the Presence of Selected Ions 16 to 21 Test Method C-Corrosivity with Magnetite-Coated Steel Specimens 22 to 28 Test Method D-Corrosivity with Deposit-Coated Specimens 29 to 35
1.2 Test Methods A and B provide for corrosivity testing under either static immersion or dynamic conditions.
1.3 Test Methods C and D are procedures applicable for corrosivity testing under static immersion conditions only.
1.4 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: D 3263 – 82 (Reapproved 1999)
Standard Test Methods for
Corrosivity of Solvent Systems for Removing Water-Formed
Deposits
This standard is issued under the fixed designation D 3263; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—A footnote was editorially removed in June 1999.
1. Scope to react with and remove deposits.
1.1 These test methods cover the determination of corrosiv-
4. Summary of Test Methods
ity of solvent systems used to remove water-formed deposits
4.1 These test methods consist of procedures wherein the
from the metal and alloy surfaces of water handling equipment.
corrosivity of solvent systems is determined by the weight loss
Four test methods are given as follows:
experienced by metal specimens during exposure to the solvent
Sections
systems.
Test Method A—Corrosivity in the Absence of Deposits 10 to 15
Test Method B—Corrosivity in the Presence of Selected Ions 16 to 21
4.2 Test Method A is a procedure to determine corrosivity
Test Method C—Corrosivity with Magnetite-Coated Steel
from the weight loss of metal specimens during exposure to
Specimens 22 to 28
solvent systems in the absence of deposits. This corrosivity can
Test Method D—Corrosivity with Deposit-Coated Specimens 29 to 35
be determined by either static immersion or dynamic tech-
1.2 Test Methods A and B provide for corrosivity testing
niques.
under either static immersion or dynamic conditions.
4.3 Test Method B provides the determination in solvent
1.3 Test Methods C and D are procedures applicable for
systems that have had selected ions added. It describes tech-
corrosivity testing under static immersion conditions only.
niques for manufacturing the solvent with the desired ions and
1.4 This standard does not purport to address all of the
refers to Test Method A for the acutal corrosivity testing.
safety concerns, if any, associated with its use. It is the
4.4 Test Method C describes the techniques used to produce
responsibility of the user of this standard to establish appro-
magnetited specimens that are subsequently used for the
priate safety and health practices and determine the applica-
determination of the corrosivity of the solvent system.
bility of regulatory limitations prior to use.
4.5 Test Method D describes the techniques used to produce
specimens coated with a synthetic deposit that are subsequently
2. Referenced Documents
used for the determination of the corrosivity of the solvent
2.1 ASTM Standards:
system.
D 1129 Terminology Relating to Water
D 1193 Specification for Reagent Water
5. Significance and Use
D 2790 Test Methods of Analysis of Solvent Systems Used
5.1 Test Method A is necessary because the corrosivity of a
for Removal of Water-Formed Deposits
solvent system can be detrimental to the equipment being
cleaned. It is used to compare the corrosivity of various solvent
3. Terminology
systems and to determine the corrosivity of selected solvent
3.1 Definitions:
systems under different conditions.
3.1.1 For definitions of terms used in these test methods,
5.2 Test Method B is necessary because the corrosivity of a
refer to Definitions D 1129.
solvent system can be changed by the presence of ions in the
3.2 Definitions of Terms Specific to This Standard:
solvent system. It is used to determine if the ions that might be
3.2.1 solvent system—specified chemicals or combinations
present during a cleaning operation would significantly change
of chemicals, which may include corrosion inhibitors designed
the corrosivity of a solvent system.
5.3 Test Method C is necessary because the corrosivity of a
These test methods are under the jurisdiction of ASTM Committee D-19 on
solvent system under magnetite removal conditions can be
Water and are the direct responsibility of Subcommittee D19.03 on Sampling of
different from the corrosivity in the absence of deposit. It is
Water and Water-Formed Deposits, Surveillance of Water, and Flow Measurement
used to determine the corrosivity of the solvent system under
of Water.
Current edition approved May 28, 1982. Published October 1982. Originally
magnetite removal conditions.
published as D 3263 – 73 T. Last previous edition D 3263 – 77.
5.4 Test Method D is necessary because the presence or
Annual Book of ASTM Standards, Vol 11.01.
absence of deposits may affect the corrosivity of the solvent
Annual Book of ASTM Standards, Vol 11.02.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3263
system. It is used to determine the corrosivity of solvent store in a desiccator until ready for use. Recheck after storage
systems on deposit-coated specimens. These results are com- for constant weight.
pared with results obtained from Test Method A to determine 7.3 Rod-Type Specimens:
the effect of the deposit. 7.3.1 Size specimens to 12.7-mm (0.5-in.) diameter with the
end rounded to a 6.35-mm (0.25-in.) radius. Perform all cutting
6. Specimen Composition and Size
and sizing operations by lathe turning, grinding, or milling,
with adequate cooling to prevent metallurgical changes due to
6.1 Test specimens for Test Methods A, B, and C may be flat
excessive heating. Perform final mechanical finishing of the
coupons, either rectangular or circular in shape, or rod or
specimen with 120-grit silicon carbide cloth.
tubular material. Regardless of form, finish all specimens to a
2 2
7.3.2 Drill a 3.2-mm (0.125-in.) hole into the axial center
size, including edges or ends, of 38.7 cm (6 in. ). Prepare
line of one end and attach a polypropylene rod section for
coupon or rod specimens from hot- or cold-rolled stock, either
suspension of the specimen in the solvent with epoxy cement.
ferrous or nonferrous, having a composition acceptable to all
7.3.3 Mark the specimens for identification as in 7.2.3.
interested parties. Take tubular specimens from cold-drawn
7.4 Tubular Specimens:
stock of appropriate composition; the inside diameter shall be
7.4.1 Perform all cutting operations by lathe turning, saw-
no less than 12.7 mm (0.5 in.). Steel specimens only are used
ing, reaming, etc. with adequate cooling to prevent metallur-
in Test Method C.
gical changes. Finish both the external and internal surfaces of
6.2 Circular coupon specimens only are used in Test Method
the tubing. Do final mechanical finishing of the specimen with
D. They shall conform to all conditions prescribed in 6.1
120-grit silicon carbide cloth.
except that the size shall be such that one side provides the test
2 2
7.4.2 Drill a 3.2-mm (0.125-in.) hole in each specimen near
area of 38.7 cm (6 in. ).
one end for suspension in the solvent.
7.4.3 Mark the specimens for identification as in 7.2.3.
7. Specimen Preparation
7.4.4 Clean and weigh specimens as specified in 7.2.4.
7.1 Prepare four specimens of whatever form for each test
condition. 8. Reagents and Materials
7.2 Coupon Specimens:
8.1 Purity of Reagents:
7.2.1 Cut specimens by sawing, abrasive cut-off, or milling
8.1.1 All solvent materials such as acids, inhibitors, and
(shearing is not permissible). Any such power-cutting opera-
other additives shall be of commercial or technical grade, such
tion must include adequate cooling to prevent metallurgical
as would normally be employed in chemical cleaning practices
changes that might result from excessive heating. Perform final
for the removal of water-formed deposits.
mechanical finishing of the specimens, with 120-grit silicon
8.1.2 Reagent grade chemicals shall be used for cleaning
carbide cloth. Round all edges and corners lightly. Sand
test specimens, for addition of selected ions to solvent systems
blasting for finishing is not permissible. For ferrous specimens
(Test Method B), preparing synthetic deposits (Test Method
the alternative use of microglass bead blasting is permissible.
D), or analyzing a solvent for active components or water-
7.2.2 Drill a 3.2-mm (0.125-in.) hole near the top of
formed deposit constitutents. Unless otherwise indicated, it is
rectangular specimens and through the center of circular test
intended that all reagents shall conform to the specifications of
pieces for suspension in the solvent.
the Committee on Analytical Reagents of the American Chemi-
7.2.3 Mark specimens for identification by an engraving
cal Society, where such specifications are available.
tool. (Do not identify specimens by stamping.)
8.2 Purity of Water— Unless otherwise indicated, refer-
7.2.4 Final preparation of the specimens shall be as follows:
ences to water shall be understood to mean reagent water,
7.2.4.1 Ferrous Specimens:
conforming to Specification D 1193, Type III.
(1) Degrease by immersion in acetone.
8.3 Reagents for Cleaning Specimens:
(2) Pickle with uninhibited hydrochloric acid (HCl, 1 + 1) at
8.3.1 Acetone (CH COCH ).
3 3
room temperature for 10 min.
8.3.2 Hydrochloric Acid (1 + 1)—Carefully add 1 volume
(3) Neutralize by immersion in hot saturated sodium bicar-
of hydrochloric acid (HCl, sp gr 1.19) to volume of water.
bonate (NaHCO ) solution.
8.3.3 Methyl Alcohol (CH OH), absolute.
(4) Rinse with water.
8.3.4 Sodium Bicarbonate Solution (100 g/L)—Dissolve
(5) Dry with acetone.
100 g of sodium bicarbonate (NaHCO ) in water and dilute
7.2.4.2 Nonferrous and Stainless Steel Specimens:
solution to 1 L.
(1) Degrease by immersion in acetone.
9. Hazards
(2) Scrub with household cleanser containing no oxidizing
9.1 This standard may involve the use of hazardous mate-
agents.
rials, operations, and equipment. It is the responsibility of
(3) Rinse with water.
(4) Dry with acetone.
7.2.4.3 Handle the specimens only with tongs or suitable
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
plastic gloves during this cleaning and drying period as well as
listed by the American Chemical Society, see Analar Standards for Laboratory
all other operations until after the final weighing following
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
exposure to the test solvent.
and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
7.2.4.4 Weigh each specimen to 61.0 mg after cooling and MD.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 3263
whoever uses this standard to establish appropriate safety and supports for the test containers.
practices and to determine the applicability of regulatory 12.1.2 Test Containers— 500-mL, tall form, lipless
limitations prior to use. polypropylene beakers. They shall be fitted with tight covers.
12.2 For Testing Under Fluid Flow Velocity Conditions:
TEST METHOD A—CORROSIVITY IN THE
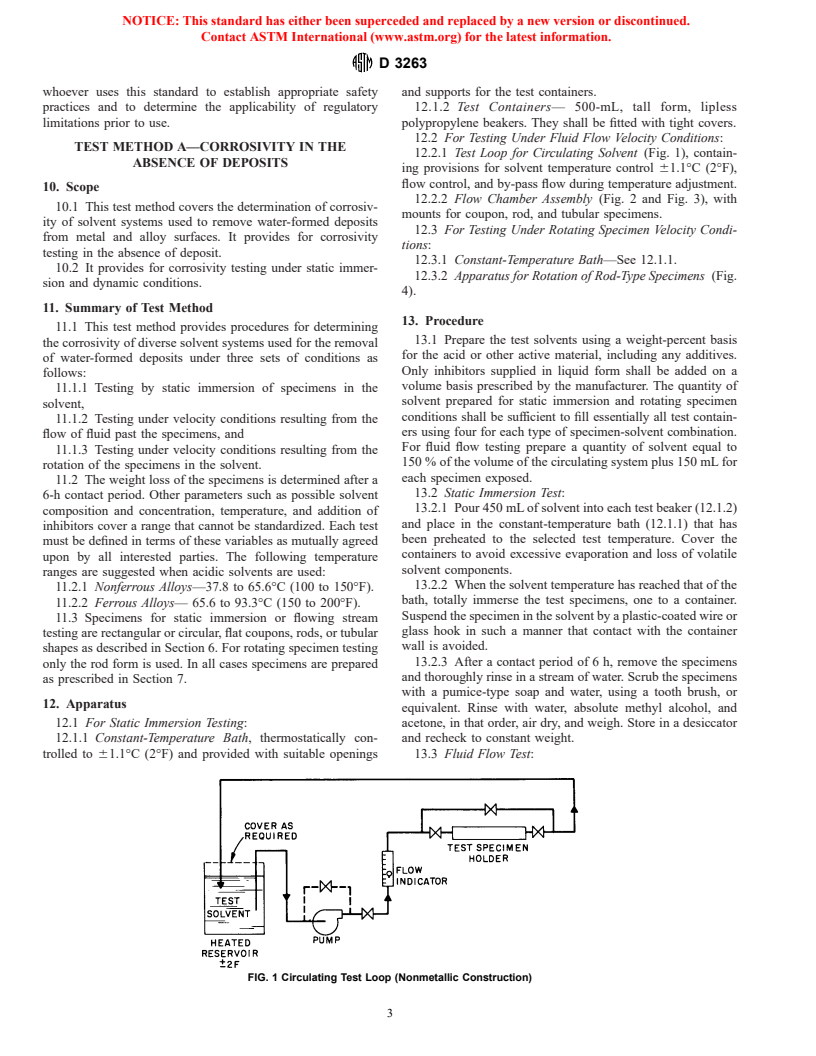
12.2.1 Test Loop for Circulating Solvent (Fig. 1), contain-
ABSENCE OF DEPOSITS
ing provisions for solvent temperature control 61.1°C (2°F),
flow control, and by-pass flow during temperature adjustment.
10. Scope
12.2.2 Flow Chamber Assembly (Fig. 2 and Fig. 3), with
10.1 This test method covers the determination of corrosiv-
mounts for coupon, rod, and tubular specimens.
ity of solvent systems used to remove water-formed deposits
12.3 For Testing Under Rotating Specimen Velocity Condi-
from metal and alloy surfaces. It provides for corrosivity
tions:
testing in the absence of deposit.
12.3.1 Constant-Temperature Bath—See 12.1.1.
10.2 It provides for corrosivity testing under static immer-
12.3.2 Apparatus for Rotation of Rod-Type Specimens (Fig.
sion and dynamic conditions.
4).
11. Summary of Test Method
13. Procedure
11.1 This test method provides procedures for determining
13.1 Prepare the test solvents using a weight-percent basis
the corrosivity of diverse solvent systems used for the removal
for the acid or other active material, including any additives.
of water-formed deposits under three sets of conditions as
Only inhibitors supplied in liquid form shall be added on a
follows:
volume basis prescribed by the manufacturer. The quantity of
11.1.1 Testing by static immersion of specimens in the
solvent prepared for static immersion and rotating specimen
solvent,
conditions shall be sufficient to fill essentially all test contain-
11.1.2 Testing under velocity conditions resulting from the
ers using four for each type of specimen-solvent combination.
flow of fluid past the specimens, and
For fluid flow testing prepare a quantity of solvent equal to
11.1.3 Testing under velocity conditions resulting from the
150 % of the volume of the circulating system plus 150 mL for
rotation of the specimens in the solvent.
each specimen exposed.
11.2 The weight loss of the specimens is determined after a
13.2 Static Immersion Test:
6-h contact period. Other parameters such as possible solvent
13.2.1 Pour 450 mL of solvent into each test beaker (12.1.2)
composition and concentration, temperature, and addition of
and place in the constant-temperature bath (12.1.1) that has
inhibitors cover a range that cannot be standardized. Each test
been preheated to the selected test temperature. Cover the
must be defined in terms of these variables as mutually agreed
containers to avoid excessive evaporation and loss of volatile
upon by all interested parties. The following temperature
solvent components.
ranges are suggested when acidic solvents are used:
13.2.2 When the solvent temperature has reached that of the
11.2.1 Nonferrous Alloys—37.8 to 65.6°C (100 to 150°F).
bath, totally immerse the test specimens, one to a container.
11.2.2 Ferrous Alloys— 65.6 to 93.3°C (150 to 200°F).
Suspend the specimen in the solvent by a plastic-coated wire or
11.3 Specimens for static immersion or flowing stream
glass hook in such a manner that contact with the container
testing are rectangular or circular, flat coupons, rods, or tubular
wall is avoided.
shapes as described in Section 6. For rotating specimen testing
13.2.3 After a contact period of 6 h, remove the specimens
only the rod form is used. In all cases specimens are prepared
and thoroughly rinse in a stream of water. Scrub the specimens
as prescribed in Section 7.
with a pumice-type soap and water, using a tooth brush, or
12. Apparatus
equivalent. Rinse with water, absolute methyl alcohol, and
12.1 For Static Immersion Testing: acetone, in that order, air dry, and we
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.