ASTM F560-17
(Specification)Standard Specification for Unalloyed Tantalum for Surgical Implant Applications (UNS R05200, UNS R05400)
Standard Specification for Unalloyed Tantalum for Surgical Implant Applications (UNS R05200, UNS R05400)
SCOPE
1.1 This specification covers chemical, mechanical, and metallurgical requirements for unalloyed tantalum plate, sheet, strip, bar, and wire used in the manufacture of surgical implants.
1.2 Units—The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values of the two systems may result in non-conformance with this standard.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F560 −17
Standard Specification for
Unalloyed Tantalum for Surgical Implant Applications (UNS
1
R05200, UNS R05400)
ThisstandardisissuedunderthefixeddesignationF560;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
3
1. Scope* 2.2 ISO Standards:
ISO 6892 Metallic Materials– Tensile Testing at Ambient
1.1 This specification covers chemical, mechanical, and
Temperature
metallurgical requirements for unalloyed tantalum plate, sheet,
ISO 9001 Quality Management Systems—Requirements
strip, bar, and wire used in the manufacture of surgical
ISO 13782 Implants for Surgery—Metallic Materials—
implants.
Unalloyed Tantalum for Surgical Implant Applications
1.2 Units—The values stated in either SI units or inch-
pound units are to be regarded separately as standard. The
3. Terminology
values stated in each system may not be exact equivalents;
3.1 Definitions of Terms Specific to This Standard:
therefore,eachsystemshallbeusedindependentlyoftheother.
3.1.1 lot—all material produced from the same ingot or a
Combining values of the two systems may result in non-
single powder blend with the same cross section under the
conformance with this standard.
same conditions at essentially the same time.
1.3 This international standard was developed in accor-
3.1.2 plate—a flat product more than 4.75 mm [0.1875 in.]
dance with internationally recognized principles on standard-
in thickness.
ization established in the Decision on Principles for the
3.1.3 bar—material 3.15 to 63.5 mm [0.125 to 2.5 in.] in
Development of International Standards, Guides and Recom-
diameter in round, hexagonal, or octagonal cross section
mendations issued by the World Trade Organization Technical
supplied in straight lengths.
Barriers to Trade (TBT) Committee.
3.1.4 sheet—a flat product 150 mm [6 in.] or more in width
2. Referenced Documents and from 0.13 to 4.75 mm [0.005 to 0.1875 in.] in thickness.
2
3.1.5 strip—a flat product less than 150 mm [6 in.] in width
2.1 ASTM Standards:
and from 0.13 to 4.75 mm [0.005 to 0.1875 in.] in thickness,
E8/E8M Test Methods for Tension Testing of Metallic Ma-
which may be supplied in coil.
terials
E29 Practice for Using Significant Digits in Test Data to
3.1.6 wire—material up to 3.15 mm [0.124 in.] in diameter
Determine Conformance with Specifications
furnished in coils or on spools or reels.
F981 Practice for Assessment of Compatibility of Biomate-
rials for Surgical Implants with Respect to Effect of 4. Ordering Information
Materials on Muscle and Insertion into Bone
4.1 Inquiries and orders under this specification shall in-
IEEE/ASTM SI 10 American National Standard for Use of
clude the following information:
theInternationalSystemofUnits(SI):TheModernMetric
4.1.1 Quantity (weight or number of pieces);
System
4.1.2 ASTM designation, alloy number, and date of issue;
4.1.3 Units to be used for certification (SI or inch-pound);
4.1.4 Composition designation (see 5.1);
4.1.5 Form (strip, sheet, plate, bar, wire) (see 3.1);
4.1.6 Condition (see 5.4);
1
This specification is under the jurisdiction of ASTM Committee F04 on
4.1.7 Applicable dimensions, including size, thickness,
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.12 on Metallurgical Materials.
width, and length (random, exact, multiples), or drawing
Current edition approved Nov. 1, 2017. Published November 2017. Originally
number;
ɛ1
approved in 1978. Last previous edition approved in 2013 as F560 – 13 . DOI:
4.1.8 Special tests;
10.1520/F0560-17.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F560−17
4.1.9 Special requirements; and 6.1.3 Intentional elemental additions other than those speci-
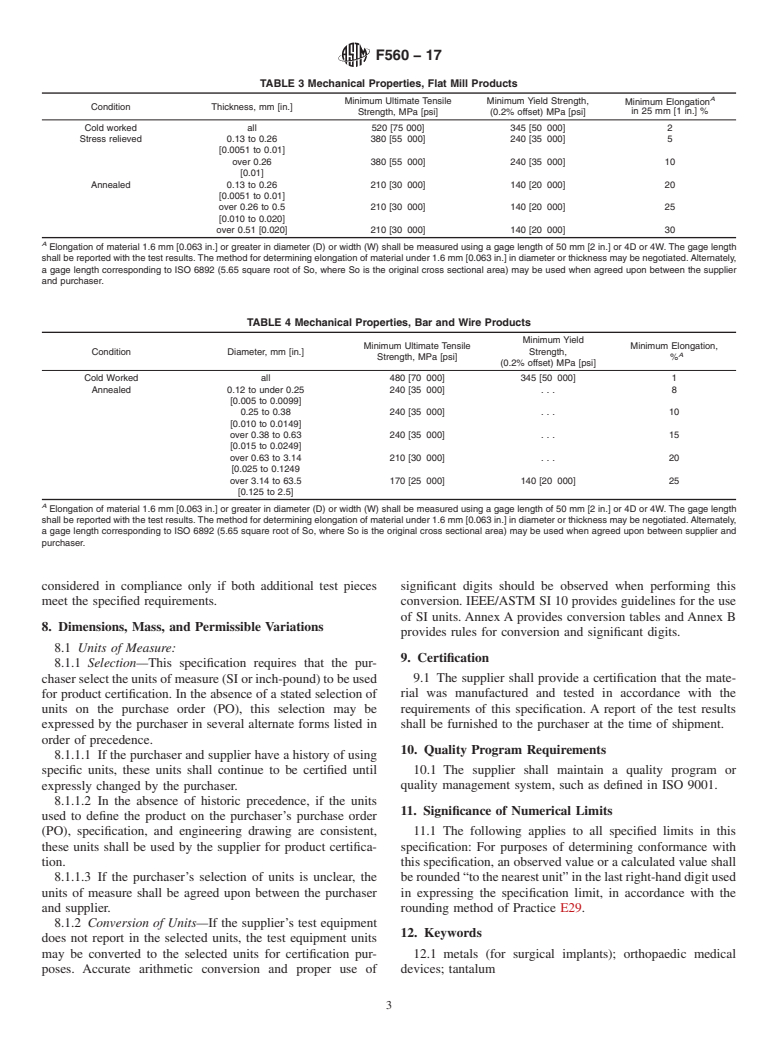
4.1.10 Mechanical properties (if applicable for special con- fied in Table 1 are not permitted
ditions) (see 7.1).
6.2 The ingot analysis shall be considered the chemical
analysis for products supplied under this specification.
5. Materials and Manufacture
6.3 When requested by the purchaser at the time of
5.1 Material covered by this specification shall be made
purch
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation: F560 − 13 F560 − 17
Standard Specification for
Unalloyed Tantalum for Surgical Implant Applications (UNS
1
R05200, UNS R05400)
This standard is issued under the fixed designation F560; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1
ε NOTE—The designation was corrected editorially in December 2013.
1. Scope*
1.1 This specification covers the chemical, mechanical, and metallurgical requirements for unalloyed tantalum plate, sheet, strip,
bar, and wire used in the manufacture of surgical implants.
1.2 TheUnits—The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values
stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining
values of the two systems may result in non-conformance with this standard.
1.3 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E8/E8M Test Methods for Tension Testing of Metallic Materials
E29 Practice for Using Significant Digits in Test Data to Determine Conformance with Specifications
F981 Practice for Assessment of Compatibility of Biomaterials for Surgical Implants with Respect to Effect of Materials on
Muscle and Insertion into Bone
IEEE/ASTM SI 10 American National Standard for Use of the International System of Units (SI): The Modern Metric System
3
2.2 ISO Standards:
ISO 6892 Metallic MaterialsMaterials– Tensile Testing at Ambient Temperature
ISO 9001 Quality Management Systems—Requirements
ISO 13782 Implants for Surgery—Metallic Materials—Unalloyed Tantalum for Surgical Implant Applications
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 lot—all material produced from the same ingot or a single powder blend with the same cross section under the same
conditions at essentially the same time.
3.1.2 plate—a flat product more than 4.75 mm [0.1875 in.] in thickness.
3.1.3 bar—material 3.15 to 63.5 mm [0.125 to 2.5 in.] in diameter in round, hexagonal, or octagonal cross section supplied in
straight lengths.
3.1.4 sheet—a flat product 150 mm [6 in.] or more in width and from 0.13 to 4.75 mm [0.005 to 0.1875 in.] in thickness.
3.1.5 strip—a flat product less than 150 mm [6 in.] in width and from 0.13 to 4.75 mm [0.005 to 0.1875 in.] in thickness, which
may be supplied in coil.
3.1.6 wire—material up to 3.15 mm [0.124 in.] in diameter furnished in coils or on spools or reels.
1
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.12 on Metallurgical Materials.
Current edition approved Oct. 1, 2013Nov. 1, 2017. Published November 2013November 2017. Originally approved in 1978. Last previous edition approved in 20082013
ɛ1
as F560 – 08.F560 – 13 . DOI: 10.1520/F0560-13E01.10.1520/F0560-17.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F560 − 17
4. Ordering Information
4.1 Inquiries and orders under this specification shall include the following information:
4.1.1 Quantity (weight or number of pieces),pieces);
4.1.2 ASTM designation, alloy number, and date of issue,issue;
4.1.3 Units to be used for certification—SI certification (SI or inch-pound.inch-pound);
4.1.4 Composition designation (see 5.1));
4.1.5 Form (strip, sheet, plate, bar, wire) (see 3.1),);
4.1.6 Condition (see 5.4),);
4.1.7 Applicable dimensions, including size, thickness, width, and length (random, exact, mu
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.