ASTM D3908-03(2008)
(Test Method)Standard Test Method for Hydrogen Chemisorption on Supported Platinum Catalysts by Volumetric Vacuum Method
Standard Test Method for Hydrogen Chemisorption on Supported Platinum Catalysts by Volumetric Vacuum Method
SIGNIFICANCE AND USE
This test method sets forth a procedure by which duplicate catalyst samples can be compared either on an interlaboratory or intralaboratory basis. It is anticipated that catalyst producers and users will find this test method of value.
Discrimination of the samples for which this procedure is recommended must be exercised when considering carrier (support) materials that sorb appreciable quantities of hydrogen or could cause an alteration of the state of the catalyst during pretreatment, or both, (that is, sintering or metal occlusion). These materials must be identified by the user and experimented with to determine the most significant conditions of measurement.
This test method provides a measure of the total hydrogen uptake (volume of hydrogen at STP, cm3/g of catalyst) without specifying the nature of the hydrogen-platinum interaction. Persons interested in using hydrogen uptake data to calculate percent platinum dispersion in a specific catalyst should be aware of carrier (support) interactions, spillover effects, and other phenomena related to the hydrogen uptake capabilities of the catalyst in question.
SCOPE
1.1 This test method covers the determination of the chemisorption of hydrogen at 298 K (25°C) on supported platinum catalysts that have been reduced in flowing hydrogen at 723 K (450°C). It incorporates a static volumetric vacuum technique at constant volume.
1.2 The test method is intended for use on unused supported platinum on alumina catalysts of loadings greater than 0.3 weight %. Data on other supports and lower platinum loadings were not tested.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3908 − 03(Reapproved 2008)
Standard Test Method for
Hydrogen Chemisorption on Supported Platinum Catalysts
by Volumetric Vacuum Method
This standard is issued under the fixed designation D3908; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
P = pressure of gas in calibrated bulb, torr
1.1 This test method covers the determination of the
c
P = pressure of gas in calibrated bulb and
mc
chemisorption of hydrogen at 298 K (25°C) on supported
manifold, torr
platinum catalysts that have been reduced in flowing hydrogen
P = pressure in manifold, torr
m
at 723 K (450°C). It incorporates a static volumetric vacuum
P = pressure in manifold and dead space, torr
md
technique at constant volume.
P = pressure in manifold prior to expansion into
m
x
1.2 Thetestmethodisintendedforuseonunusedsupported
sample tube for X equilibration point, torr
platinum on alumina catalysts of loadings greater than 0.3
P = equilibrium pressure after expansion for gen-
e
x
weight %. Data on other supports and lower platinum loadings
erating X equilibrium point, torr
were not tested.
V = volume of calibrated bulb, cm
c
V = volume of manifold between stopcocks 12
m
1.3 This standard does not purport to address all of the
and 2 with only 4 and 1 open, cm
safety concerns, if any, associated with its use. It is the
V = volume of dead space in sample cell contain-
d
responsibility of the user of this standard to establish appro-
ing catalyst (volume between 2 and 3), cm
priate safety and health practices and determine the applica-
V (STP) = volume of gas adsorbed at STP, cm
ads x
bility of regulatory limitations prior to use.
V (STP) = cumulative volume of gas adsorbed through
ads cx
X,cm
2. Referenced Documents
V = monolayer volume of gas adsorbed at STP,
2 S
2.1 ASTM Standards:
cm
D3766Terminology Relating to Catalysts and Catalysis
T = temperature representative of the manifold
m
Ax
E177Practice for Use of the Terms Precision and Bias in
prior to expansion into the sample cell, K
ASTM Test Methods
T = temperature representative of the entire sys-
m
Bx
E456Terminology Relating to Quality and Statistics
tem after equilibrium pressure (P ) has been
e
x
E691Practice for Conducting an Interlaboratory Study to
established, K
Determine the Precision of a Test Method
T = temperature of manifold prior to expansion
m
intosamplecellfordeadspacedetermination,
3. Terminology
K
3.1 Definitions—See Terminology D3766.
T = temperatureofentiresystemafterequilibrium
m
D
pressure has been established for dead space
3.2 Quality and Statistics—See Terminology E456.
determination, K
3.3 Precision and Bias—See Practice E177.
T = average manifold temperature for a given
dose, K
3.4 Symbols—The following symbols are used:
=(T + T )/2
m m
Ax Bx
W = mass of catalyst, g
cat
This test method is under the jurisdiction of ASTM Committee D32 on
X = weight percent of platinum
Catalysts and is the direct responsibility of Subcommittee D32.01 on Physical-
%D = percent platinum atoms on the surface
Chemical Properties.
Current edition approved April 1, 2008. Published May 2008. Originally
4. Significance and Use
approved in 1980. Last previous edition approved in 2003 as D3908–03. DOI:
10.1520/D3908-03R08.
4.1 This test method sets forth a procedure by which
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
duplicate catalyst samples can be compared either on an
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
interlaboratory or intralaboratory basis. It is anticipated that
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. catalystproducersanduserswillfindthistestmethodofvalue.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3908 − 03 (2008)
FIG. 1 Schematic: Static Vacuum System
4.2 Discrimination of the samples for which this procedure
is recommended must be exercised when considering carrier
(support)materialsthatsorbappreciablequantitiesofhydrogen
or could cause an alteration of the state of the catalyst during
pretreatment, or both, (that is, sintering or metal occlusion).
These materials must be identified by the user and experi-
mented with to determine the most significant conditions of
measurement.
4.3 This test method provides a measure of the total
hydrogen uptake (volume of hydrogen at STP, cm /g of
catalyst) without specifying the nature of the hydrogen-
platinum interaction. Persons interested in using hydrogen
uptake data to calculate percent platinum dispersion in a
specific catalyst should be aware of carrier (support)
interactions, spillover effects, and other phenomena related to
the hydrogen uptake capabilities of the catalyst in question.
5. Apparatus
5.1 Gas-Handling System, as shown in Fig. 1. The compo-
nents may be either glass or metal. Commercial metal instru-
ments are available. The following components are to be
included in the glass system:
5.1.1 Vacuum System, capable of attaining pressures below
−5
1 mPa (1×10 torr). The vacuum can be monitored with any
suitablevacuumgage.Adiffusionpumpbackedbyamechani-
cal pump must be isolated from the system by a trap held at
liquid nitrogen temperature. High-vacuum stopcocks using a
low-vapor pressure grease can be employed.
5.1.2 Pressure-Measuring Device, that operates at constant
volume and that is capable of reading in the range from 0 to
66.7 kPa (0 to 500 torr) to the nearest 0.01 kPa (0.1 torr).
5.1.3 Calibration Bulb, whose volume has been carefully
determined to within 0.1 % prior to attachment to the main
manifold. Typically one fills the bulb and stopcock bore with
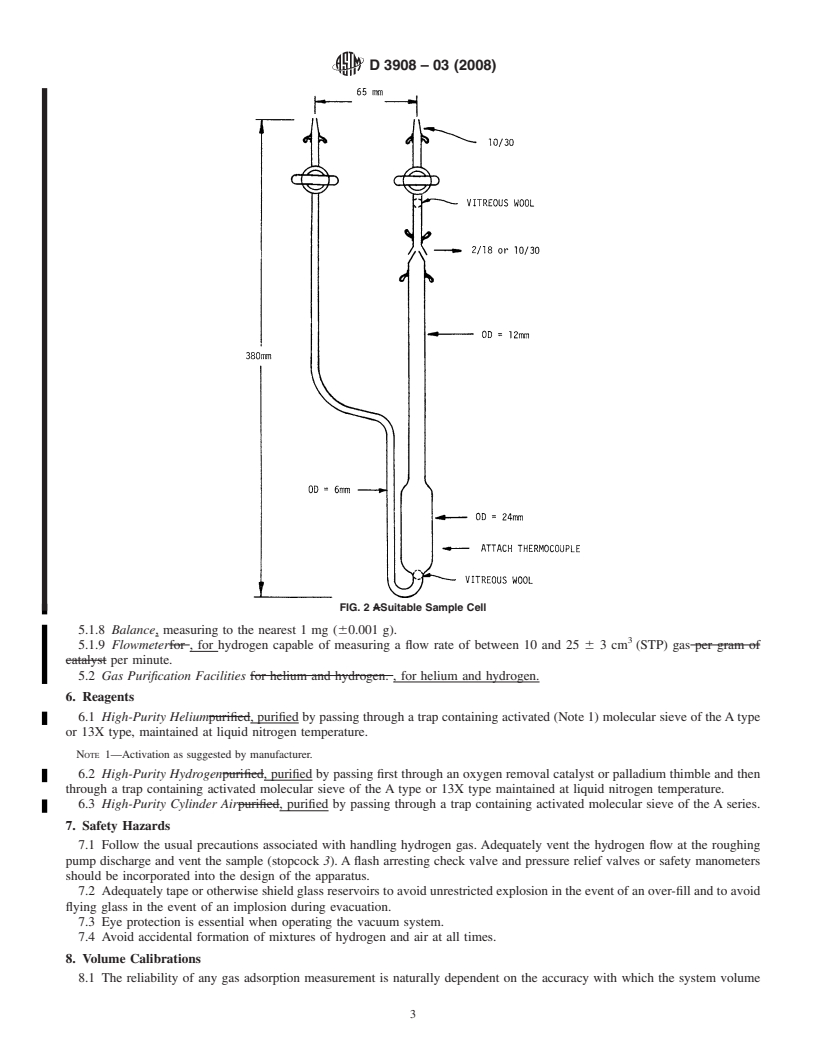
FIG. 2 Suitable Sample Cell
mercury, weighs it, and calculates the volume of the bulb from
the density of mercury at the temperature of the measurement.
Following careful cleaning, the bulb is attached to the main
manifold. One should make sure that the glass blowing is fittings, and so forth, are suitable.) Its mate is attached to the
sufficiently far removed from the calibrated volume to avoid main manifold by a glass vacuum stopcock.Astopcock is also
distortion. included on the vent side of the cell to allow for vacuum and
5.1.4 Flow-Through Cell,thatcanbeevacuatedandthatcan flow-through procedures.
be detached from the main manifold as, for example, see Fig. 5.1.5 Catalyst Sample, secured by a quartz wool plug
2. This is accomplished by including a removable joint, if upstream of the catalyst and another quartz wool plug down-
glass,amaleconejoint,onthemanifoldendofthetube.(Other stream (Fig. 2). The sample should be in the form of an
types of joints, that is, Swagelok with TFE-fluorocarbon extrudate, pellets, or powder greater than 20 mesh.
D3908 − 03 (2008)
5.1.6 Furnace, capable of maintaining a heating rate of 8.1.2 Close stopcocks 12 and 8 and introduce helium to the
5K⁄min and a temperature-control mechanism capable of system by opening 6.After establishment of a pressure of less
maintaining the furnace at temperatures in the range from 673 than one atmosphere, close 6. Record the pressure (P ).
c
to 773 6 10 K (400 to 500°C). 8.1.3 Close stopcock 5, open 12, and evacuate.
5.1.7 Thermometer or Thermocouple, to monitor the fur- 8.1.4 Close stopcock 12 and expand gas in the calibration
nace temperature to within 65 K and two thermometers to bulb by opening 5. Record final pressure (P ).
mc
registerthetemperatureofthemanifoldsystemandsamplecell 8.1.5 Repeat Steps 8.1.2 – 8.1.4 ten times and average to
during uptake determination to the nearest 60.1 K. obtain the final pressure (P ).
mc
5.1.8 Balance, measuring to the nearest 1 mg (60.001 g).
9. Charging Sample
5.1.9 Flowmeter, for hydrogen capable of measuring a flow
9.1 The amount of sample to be charged is determined by
rate of between 10 and 25 63cm (STP) gas per minute.
the expected hydrogen uptake and the maximum capacity of
5.2 Gas Purification Facilities , for helium and hydrogen.
the sample cell. Experience and the platinum loading will
dictate the optimum amount, but a minimum of1gis
6. Reagents
considered essential. This mass need not be precisely known
6.1 High-Purity Helium, purified by passing through a trap
since a final weighing will be made after determination of the
containing activated (Note 1) molecular sieve of theAtype or
hydrogen uptake. It may, however, be useful for the determi-
13X type, maintained at liquid nitrogen temperature.
nationofvolatileorcombustiblematterpresent,orboth,onthe
unused catalyst.
NOTE 1—Activation as suggested by manufacturer.
9.1.1 Plugs of quartz wool are to be charged to the cell as
6.2 High-Purity Hydrogen, purified by passing first through
shown in Fig. 2. Weigh the cell and wool plug(s).
an oxygen removal catalyst or palladium thimble and then
9.1.2 Charge at least1gof catalyst to the cell.
through a trap containing activated molecular sieve of the A
9.1.3 Connect the cell to the main manifold at stopcock 2
type or 13X
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately,ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D3908–99 Designation:D3908–03 (Reapproved 2008)
Standard Test Method for
Hydrogen Chemisorption on Supported Platinum on
Alumina Catalysts and Catalyst Carriers by Volumetric
Vacuum MethodHydrogen Chemisorption on Supported
Platinum Catalysts by Volumetric Vacuum Method
This standard is issued under the fixed designation D3908; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the determination of the chemisorption of hydrogen at 298 K (25°C) on supported platinum
catalysts that have been reduced in flowing hydrogen at 723 K (450°C). It incorporates a static volumetric vacuum technique at
constant volume.
1.2 The test method is intended for use on unused supported platinum on alumina catalysts of loadings greater than 0.3 weight
%. Data on other supports and lower platinum loadings were not tested.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D3766 Terminology Relating to Catalysts and Catalysis
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E456 Terminology Relating to Quality and Statistics
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
3. Symbols
3.1The following symbols are used: Terminology
3.1 Definitions—See Terminology D3766.
3.2 Quality and Statistics—See Terminology E456.
3.3 Precision and Bias—See Practice E177.
3.4 Symbols—The following symbols are used:
P = pressure of gas in calibrated bulb, torr
c
P = pressure of gas in calibrated bulb and manifold, torr
mc
P = pressure in manifold, torr
m
P = pressure in manifold and dead space, torr
md
P = pressure in manifold prior to expansion into sample tube for X equilibration point, torr
m
x
P = equilibrium pressure after expansion for generating X equilibrium point, torr
e
x
V = volume of calibrated bulb, cm
c
V = volume of manifold between stopcocks 12 and 2 with only 4 and 1 open, cm
m
V = volume of dead space in sample cell containing catalyst (volume between 2 and 3), cm
d
V (STP) = volume of gas adsorbed at STP, cm
ads x
V ( STP) = cumulative volume of gas adsorbed through X,cm
ads cx
This test method is under the jurisdiction of ASTM Committee D-32D32 on Catalysts and is the direct responsibility of Subcommittee D32.01 on Physical-Chemical
Properties.
Current edition approvedApril 10, 1999.1, 2008. Published June 1999.May 2008. Originally published as D3908–80.approved in 1980. Last previous edition D3908–88
e1
(1993) .approved in 2003 as D3908–03.
ForreferencedASTMstandards,visittheASTMwebsite,www.astm.org,orcontactASTMCustomerServiceatservice@astm.org.For Annual Book of ASTM Standards
, Vol 14.02.volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D3908–03 (2008)
V = monolayer volume of gas adsorbed at STP, cm
S
T = temperature representative of the manifold prior to expansion into the sample cell, K
m
Ax
T = temperature representative of the entire system after equilibrium pressure (P ) has been established, K
m e
Bx x
T = temperature of manifold prior to expansion into sample cell for dead space determination, K
m
T = temperature of entire system after equilibrium pressure has been established for dead space determination, K
m
D
T = average manifold temperature for a given dose, K
=(T + T )/2
m m
Ax Bx
W = weight of catalyst, gmass of catalyst, g
cat
X = weight percent of platinum
%D = percent platinum atoms on the surface
4. Significance and Use
4.1 This test method sets forth a procedure by which duplicate catalyst samples can be compared either on an interlaboratory
or intralaboratory basis. It is anticipated that catalyst producers and users will find this test method of value.
4.2 Discrimination of the samples for which this procedure is recommended must be exercised when considering carrier
(support) materials that ad(ab)sorbsorb appreciable quantities of hydrogen or could cause an alteration of the state of the catalyst
duringpretreatment,orboth,(thatis,sinteringormetalocclusion).Thesematerialsmustbeidentifiedbytheuserandexperimented
with to determine the most significant conditions of measurement.
4.3 Thistestmethodprovidesameasureofthetotalhydrogenuptake(mL(atstandardconditions(STP)H
2(volume of hydrogen at STP,
cm/gofcatalyst))withoutspecifyingthenatureofthehydrogen-platinuminteraction.Personsinterestedinusinghydrogenuptake
data to calculate percent platinum (dispersion)dispersion in a specific catalyst should be aware of carrier (support) interactions,
spill-over effects, and other phenomena related to the hydrogen uptake capabilities of the catalyst in question.
5. Apparatus
5.1 Gas-Handling Systemas shown in , as shown in Fig. 1. The components may be either glass or metal. Commercial metal
instruments are available. The following components are to be included in the glass system:
−5
5.1.1 Vacuum System, capable of attaining pressures below 1 mPa (1 310 torr). The vacuum can be monitored with any
suitable vacuum gage.Adiffusion pump backed by a mechanical pump must be isolated from the system by a trap held at liquid
nitrogen temperature. High-vacuum stopcocks using a low-vapor pressure grease can be employed.
5.1.2 Pressure-Measuring Devicethat,thatoperatesatconstantvolumeandthatiscapableofreadingintherangefrom0to66.7
kPa (0 to 500 torr) to the nearest 0.01 kPa (0.1 torr).
5.1.3 Calibration Bulb,whosevolumehasbeencarefullydeterminedtowithin0.1%priortoattachmenttothemainmanifold.
Typically one fills the bulb and stopcock bore with mercury, weighs it, and calculates the volume of the bulb from the density of
mercuryatthetemperatureofthemeasurement.Followingcarefulcleaning,thebulbisattachedtothemainmanifold.Oneshould
make sure that the glass blowing is sufficiently far removed from the calibrated volume to avoid distortion.
5.1.4 Flow-Through Cellthat, that can be evacuated and that can be detached from the main manifold as, for example, see Fig.
2.This is accomplished by including a removeable joint, if glass, a male cone joint, on the manifold end of the tube. (Other types
ofjoints,thatis,SwagelokwithTFE-fluorocarbonfittings,etc.,andsoforth,aresuitable.)Itsmateisattachedtothemainmanifold
by a glass vacuum stopcock. A stopcock is also included on the vent side of the cell to allow for vacuum and flow-through
procedures.
5.1.5 Catalyst Sample, secured by a quartz wool plug upstream of the catalyst and another quartz wool plug downstream (Fig.
2). The sample should be in the form of an extrudate, pellets, or powder greater than 20 mesh.
5.1.6 Furnacecapable , capable of maintaining a heating rate of 5 deg/min 5K/min and a temperature-control mechanism
capable of maintaining the furnace at temperatures in the range from 673 to 773 6 10 K (400 to 500°C).
5.1.7 Thermometer or Thermocouple to, to monitor the furnace temperature to within 65 K and two thermometers to register
the temperature of the manifold system and sample cell during uptake determination to the nearest 60.1 K.
FIG. 1 Schematic: Static Vacuum System
D3908–03 (2008)
FIG. 2 ASuitable Sample Cell
5.1.8 Balance, measuring to the nearest 1 mg (60.001 g).
5.1.9 Flowmeterfor , for hydrogen capable of measuring a flow rate of between 10 and 25 63cm (STP) gas per gram of
catalyst per minute.
5.2 Gas Purification Facilities for helium and hydrogen. , for helium and hydrogen.
6. Reagents
6.1 High-Purity Heliumpurified, purified by passing through a trap containing activated (Note 1) molecular sieve of theAtype
or 13X type, maintained at liquid nitrogen temperature.
NOTE 1—Activation as suggested by manufacturer.
6.2 High-Purity Hydrogenpurified, purified by passing first through an oxygen removal catalyst or palladium thimble and then
through a trap containing activated molecular sieve of the A type or 13X type maintained at liquid nitrogen temperature.
6.3 High-Purity Cylinder Airpurified, purified by passing through a trap containing activated molecular sieve of the A series.
7. Safety Hazards
7.1 Follow the usual precautions associated with handling hydrogen gas. Adequately vent the hydrogen flow at the roughing
pump discharge and vent the sample (stopcock 3).Aflash arresting check valve and pressure relief valves or safety manometers
should be incorporated into the design of the apparatus.
7.2 Adequatelytapeorotherwiseshieldglassreservoirstoavoidunrestrictedexplosionintheeventofanover-fillandtoavoid
flying glass in the event of an implosion during evacuation.
7.3 Eye protection is essential when operating the vacuum system.
7.4 Avoid accidental formation of mixtures of hydrogen and air at all times.
8. Volume Calibrations
8.1 The reliability of any gas adsorption measurement is naturally dependent on the accuracy with which the system volume
---------
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.