ASTM D2036-09(2022)
(Test Method)Standard Test Methods for Cyanides in Water
Standard Test Methods for Cyanides in Water
SIGNIFICANCE AND USE
5.1 Cyanide is highly toxic. Regulations have been established to require the monitoring of cyanide in industrial and domestic wastes and in surface waters (Appendix X1).
5.2 Test Method D is applicable for natural water and clean metal finishing or heat treatment effluents. It may be used for process control in wastewater treatment facilities providing its applicability has been validated by Test Method B or C.
5.3 The spot test outlined in Annex A1 can be used to detect cyanide and thiocyanate in water or wastewater, and to approximate its concentration.
SCOPE
1.1 These test methods cover the determination of cyanides in water. The following test methods are included:
Sections
Test Method A
Total Cyanides after Distillation
12 – 18
Test Method B
Cyanides Amenable to Chlorination2
by Difference
19 – 25
Test Method C
Weak Acid Dissociable Cyanides
26 – 32
Test Method D
Cyanides Amenable to Chlorination without
Distillation (Short-Cut Method)
33 – 39
1.2 Cyanogen halides may be determined separately.
Note 1: Cyanogen chloride is the most common of the cyanogen halide complexes as it is a reaction product and is usually present when chlorinating cyanide-containing industrial waste water. For the presence or absence of CNCl, the spot test method given in Annex A1 can be used.
1.3 These test methods do not distinguish between cyanide ions and metallocyanide compounds and complexes. Furthermore, they do not detect the cyanates. Cyanates can be determined using ion chromatography without digestion.
Note 2: The cyanate complexes are decomposed when the sample is acidified in the distillation procedure.
1.4 The cyanide in cyanocomplexes of gold, platinum, cobalt and some other transition metals is not completely recovered by these test methods. Refer to Test Method D6994 for the determination of cyanometal complexes.
1.5 Cyanide from only a few organic cyanides are recovered, and those only to a minor extent.
1.6 Part or all of these test methods have been used successfully with reagent water and various waste waters. It is the user's responsibility to assure the validity of the test method for the water matrix being tested.
1.7 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. Specific hazard statements are given in 5.1, 8.8, 8.18, Section 9, 11.3, and 16.1.9.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D2036 − 09 (Reapproved 2022)
Standard Test Methods for
Cyanides in Water
This standard is issued under the fixed designation D2036; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope the user’s responsibility to assure the validity of the test
method for the water matrix being tested.
1.1 These test methods cover the determination of cyanides
in water. The following test methods are included: 1.7 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
Sections
Test Method A 12–18
standard.
Total Cyanides after Distillation
1.8 This standard does not purport to address all of the
Test Method B 19–25
Cyanides Amenable to Chlorination
safety concerns, if any, associated with its use. It is the
by Difference
responsibility of the user of this standard to establish appro-
Test Method C 26–32
priate safety, health, and environmental practices and deter-
Weak Acid Dissociable Cyanides
Test Method D 33–39
mine the applicability of regulatory limitations prior to use.
Cyanides Amenable to Chlorination without
Specific hazard statements are given in 5.1, 8.8, 8.18, Section
Distillation (Short-Cut Method)
9, 11.3, and 16.1.9.
1.2 Cyanogen halides may be determined separately.
1.9 This international standard was developed in accor-
NOTE 1—Cyanogen chloride is the most common of the cyanogen dance with internationally recognized principles on standard-
halide complexes as it is a reaction product and is usually present when
ization established in the Decision on Principles for the
chlorinating cyanide-containing industrial waste water. For the presence
Development of International Standards, Guides and Recom-
or absence of CNCl, the spot test method given in AnnexA1 can be used.
mendations issued by the World Trade Organization Technical
1.3 These test methods do not distinguish between cyanide
Barriers to Trade (TBT) Committee.
ions and metallocyanide compounds and complexes.
Furthermore, they do not detect the cyanates. Cyanates can be
2. Referenced Documents
determined using ion chromatography without digestion.
2.1 ASTM Standards:
NOTE 2—The cyanate complexes are decomposed when the sample is D1129Terminology Relating to Water
acidified in the distillation procedure.
D1193Specification for Reagent Water
D2777Practice for Determination of Precision and Bias of
1.4 The cyanide in cyanocomplexes of gold, platinum,
cobalt and some other transition metals is not completely Applicable Test Methods of Committee D19 on Water
D5788Guide for Spiking Organics into Aqueous Samples
recovered by these test methods. Refer to Test Method D6994
for the determination of cyanometal complexes. D5847Practice for Writing Quality Control Specifications
for Standard Test Methods for Water Analysis
1.5 Cyanide from only a few organic cyanides are
D6696Guide for Understanding Cyanide Species
recovered, and those only to a minor extent.
D6888Test Method for Available Cyanides with Ligand
1.6 Part or all of these test methods have been used
DisplacementandFlowInjectionAnalysis(FIA)Utilizing
successfully with reagent water and various waste waters. It is
Gas Diffusion Separation and Amperometric Detection
D6994Test Method for Determination of Metal Cyanide
Complexes in Wastewater, Surface Water, Groundwater
and Drinking Water Using Anion Exchange Chromatog-
These test methods are under the jurisdiction of ASTM Committee D19 on
Water and are the direct responsibility of Subcommittee D19.06 on Methods for
raphy with UV Detection
Analysis for Organic Substances in Water.
D7284Test Method for Total Cyanide in Water by Micro
Current edition approved May 1, 2022. Published May 2022. Originally
approved in 1964. Last previous edition approved in 2015 as D2036–09(2015).
DOI: 10.1520/D2036-09R22.
2 3
For an explanation of the term cyanides amenable to alkaline chlorination, see For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Lancy, L. E. and Zabban, W., “Analytical Methods and Instrumentation for contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Determining Cyanogen Compounds,” Papers on Industrial Water and Industrial Standards volume information, refer to the standard’s Document Summary page on
Waste Water, ASTM STP 337, 1962, pp. 32–45. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D2036 − 09 (2022)
Distillation followed by Flow InjectionAnalysis with Gas 4.7 Round-robin data indicate the following minimum con-
Diffusion Separation and Amperometric Detection centrations: colorimetric 0.03 mg/L; titration 1.0 mg/L; and
D7365Practice for Sampling, Preservation and Mitigating selective ion electrode 0.03 mg/L. Ion chromatography and
Interferences in Water Samples for Analysis of Cyanide Test Method D6888 have a minimum levels equal to approxi-
E60Practice for Analysis of Metals, Ores, and Related mately 0.002 mg/L.
Materials by Spectrophotometry
5. Significance and Use
E275PracticeforDescribingandMeasuringPerformanceof
Ultraviolet and Visible Spectrophotometers
5.1 Cyanide is highly toxic. Regulations have been estab-
lished to require the monitoring of cyanide in industrial and
3. Terminology
domestic wastes and in surface waters (Appendix X1).
3.1 Definitions:
5.2 Test Method D is applicable for natural water and clean
3.1.1 For definitions of terms used in this standard, refer to
metal finishing or heat treatment effluents. It may be used for
Terminology D1129 and Guide D6696.
process control in wastewater treatment facilities providing its
applicability has been validated by Test Method B or C.
3.2 Acronyms:
3.2.1 FIA, n—flow injection analysis
5.3 ThespottestoutlinedinAnnexA1canbeusedtodetect
cyanide and thiocyanate in water or wastewater, and to
3.2.2 HPLC, n—high performance liquid chromatography
approximate its concentration.
3.2.3 IC, n—ion chromatography
3.2.4 PAD, n—pulsed amperometric detection 6. Interferences
6.1 Common interferences in the analysis for cyanide in-
4. Summary of Test Method
clude oxidizing agents, sulfides, aldehydes, glucose and other
4.1 The cyanide as hydrocyanic acid (HCN) is released sugars, high concentration of carbonate, fatty acids,
from compounds by means of reflux distillation and absorbed thiocyanate, and other sulfur containing compounds.
in sodium hydroxide solution. The conditions used for the
6.2 It is beyond the scope of these test methods to describe
distillationdistinguishthetypeofcyanide.Thesodiumcyanide
proceduresforovercomingallofthepossibleinterferencesthat
in the absorbing solution can be determined colorimetrically,
may be encountered. Refer to Practice D7365 for potential
by ion chromatography, titration, by selective ion electrode, or
interferences for the analysis of cyanide in water.
as described in Test Method D6888 using flow injection with
amperometric detection.
7. Apparatus
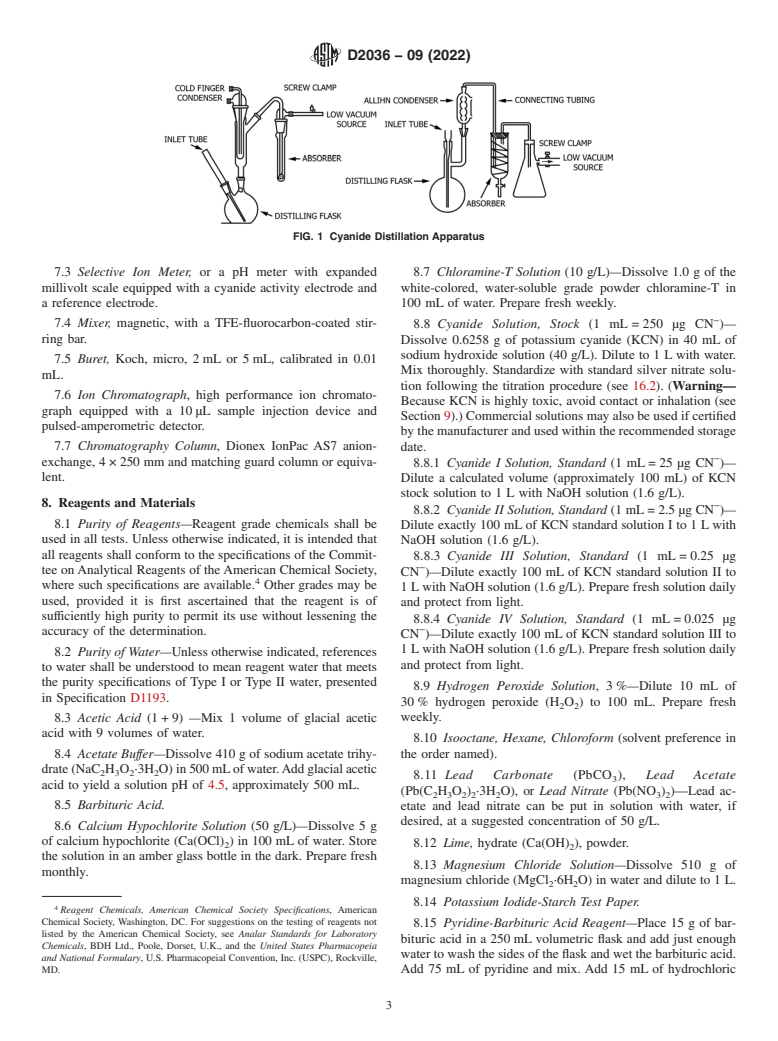
4.2 Test MethodA, Total Cyanides, is based on the decom-
7.1 Distillation Apparatus—The reaction vessel shall be a
position of nearly all cyanides in the presence of strong acid,
1L round bottom flask, with provision for an inlet tube and a
magnesium chloride catalyst, and heat during a 1-h reflux
condenser. The inlet tube shall be a funnel with an 8mm
distillation.
diameterstemthatextendstowithin6mmofthebottomofthe
flask. The condenser, which is recommended, shall be a
4.3 Test Method B, Cyanide Amenable to Chlorination, is
reflux-type, cold finger, or Allihn. The condenser shall be
based on chlorinating a portion of the sample under controlled
connected to a vacuum-type absorber which shall be in turn
conditions followed by the determination of total cyanide in
connected to a vacuum line which has provision for fine
both the original and chlorinated samples. Cyanides amenable
control. The flask shall be heated with an electric heater.
to chlorination are calculated by difference.
Examples of the apparatus are shown in Fig. 1. Equivalent
4.3.1 This test method can be affected by compounds that
apparatus is acceptable provided cyanide recoveries of 100%
are converted during chlorination to color-producing com-
6 4% are documented.
pounds or react with the reagents used, and cause interference
7.1.1 Smallerdistillationtubessuchas50mLMIDItubesor
in the procedure employed to determine cyanide in the absorp-
6mLMicroDist (trademarked) tubes described inTest Method
tion solution.
D7284 can be used if the quality control requirements in
4.4 Test Method C, Weak Acid Dissociable Cyanides, is
Section 40 are satisfied. The reagents should be added propor-
based on the decomposition of cyanides in the presence of
tionately to those specified in this test method for smaller
weakacid,zincacetateandheatduringa1-hrefluxdistillation.
sample sizes. While the use of smaller distillation tubes is
4.5 Test Method D, Cyanide Amenable to Chlorination generally accepted, the interlaboratory study was conducted
without Distillation, is a direct colorimetric procedure. with 500mL samples; therefore, the user is responsible to
determine the actual precision and bias when using a different
4.6 In the absence of interference, the minimum concentra-
type of distillation apparatus.
tionofcyanideintheabsorptionsolutionthatcanbeaccurately
determined colorimetrically is 0.005 mg/L, ion chromatogra- 7.2 Spectrophotometer or Filter Photometer, suitable for
phy and Test Method D6888 are 0.002 mg/L, titration is 0.4 measurement in the region of 578 nm, using 1.0cm, 2.0cm,
mg/Landbyselectiveionelectrodeis0.05mg/L.Pretreatment 5.0cm, and 10.0cm absorption cells. Filter photometers and
including distillation tends to increase these concentrations to photometric practices used in these test methods shall conform
a degree determined by the amount of manipulation required to Practice E60. Spectrophotometers shall conform to Practice
and the type of sample. E275.
D2036 − 09 (2022)
FIG. 1 Cyanide Distillation Apparatus
7.3 Selective Ion Meter, or a pH meter with expanded 8.7 Chloramine-T Solution (10 g/L)—Dissolve 1.0 g of the
millivolt scale equipped with a cyanide activity electrode and white-colored, water-soluble grade powder chloramine-T in
a reference electrode. 100 mL of water. Prepare fresh weekly.
−
7.4 Mixer, magnetic, with a TFE-fluorocarbon-coated stir-
8.8 Cyanide Solution, Stock (1 mL = 250 µg CN )—
ring bar.
Dissolve 0.6258 g of potassium cyanide (KCN) in 40 mL of
sodium hydroxide solution (40 g/L). Dilute to 1 L with water.
7.5 Buret, Koch, micro, 2mL or 5mL, calibrated in 0.01
Mix thoroughly. Standardize with standard silver nitrate solu-
mL.
tion following the titration procedure (see 16.2). (Warning—
7.6 Ion Chromatograph, high performance ion chromato-
Because KCN is highly toxic, avoid contact or inhalation (see
graph equipped with a 10µL sample injection device and
Section9).)Commercialsolutionsmayalsobeusedifcertified
pulsed-amperometric detector.
bythemanufacturerandusedwithintherecommendedstorage
7.7 Chromatography Column, Dionex IonPac AS7 anion- date.
−
exchange, 4×250 mm and matching guard column or equiva-
8.8.1 Cyanide I Solution, Standard(1mL=25µgCN )—
lent.
Dilute a calculated volume (approximately 100 mL) of KCN
stock solution to 1 L with NaOH solution (1.6 g/L).
8. Reagents and Materials −
8.8.2 Cyanide II Solution, Standard (1 mL=2.5 µg CN )—
8.1 Purity of Reagents—Reagent grade chemicals shall be
Dilute exactly 100 mLof KCN standard solution I to 1 Lwith
used in all tests. Unless otherwise indicated, it is intended that NaOH solution (1.6 g/L).
all reagents shall conform to the specifications of the Commit-
8.8.3 Cyanide III Solution, Standard (1 mL=0.25 µg
−
tee onAnalytical Reagents of theAmerican Chemical Society,
CN )—Dilute exactly 100 mL of KCN standard solution II to
where such specifications are available. Other grades may be
1 Lwith NaOH solution (1.6 g/L). Prepare fresh solution daily
used, provided it is first ascertained that the reagent is of
and protect from light.
sufficiently high purity to permit its use without lessening the
8.8.4 Cyanide IV Solution, Standard (1 mL=0.025 µg
−
accuracy of the determination.
CN )—Dilute exactly 100 mLof KCN standard solution III to
1 Lwith NaOH solution (1.6 g/L). Prepare fresh solution daily
8.2 Purity of Water—Unless otherwise indicated, references
and protect from light.
to water shall be understood to mean reagent water that meets
the purity specifications of Type I or Type II water, presented
8.9 Hydrogen Peroxide Solution,3%—Dilute 10 mL of
in Specification D1193.
30% hydrogen peroxide (H O ) to 100 mL. Prepare fresh
2 2
weekly.
8.3 Acetic Acid (1+9) —Mix 1 volume of glacial acetic
acid with 9 volumes of water.
8.10 Isooctane, Hexane, Chloroform (solvent preference in
the order named).
8.4 Acetate Buffer—Dissolve 410 g of sodium acetate trihy-
drate(NaC H O ·3H O)in500mLofwater.Addglacialacetic
2 3 2 2
8.11 Lead Carbonate (PbCO ), Lead Acetate
acid to yield a solution pH of 4.5, approximately 500 mL.
(Pb(C H O ) ·3H O), or Lead Nitrate (Pb(NO ) )—Lead ac-
2 3 2 2 2 3 2
8.5 Barbituric Acid.
etate and lead nitrate can be put in solution with water, if
desired, at a suggested concentration of 50 g/L.
8.6 Calcium Hypochlorite Solution (50 g/L)—Dissolve 5 g
of calcium hypochlorite (Ca(OCl) ) in 100 mL of water. Store
8.12 Lime, hydrate (Ca(OH) ), powder.
the solution in an amber glass bottle in the dark. Prepare fresh
8.13 Magnesium Chloride Solution—Dissolve 510 g of
monthly.
magnesium chloride (MgCl ·6H O) in water and dilute to 1 L.
2 2
8.14 Potassium Iodide-Starch Test Paper.
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
8.15 Pyridine-Barbituric Acid Reagent—Place 15 g of bar-
listed by the American Chemical Society, see Analar Standards for Laboratory
bituric acid in a 250mL volumetric flask and add just enough
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
watertowashthesidesoftheflaskandwetthebarbituricacid.
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
MD. Add 75 mL of pyridine and mix. Add 15 mL of hydrochloric
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.