ASTM G100-89(2010)e1
(Test Method)Standard Test Method for Conducting Cyclic Galvanostaircase Polarization
Standard Test Method for Conducting Cyclic Galvanostaircase Polarization
SIGNIFICANCE AND USE
In this test method, susceptibility to localized corrosion of aluminum is indicated by a protection potential (Eprot) determined by cyclic galvanostaircase polarization (1). The more noble this potential, the less susceptible is the alloy to initiation of localized corrosion. The results of this test method are not intended to correlate in a quantitative manner with the rate of propagation of localized corrosion that one might observe in service.
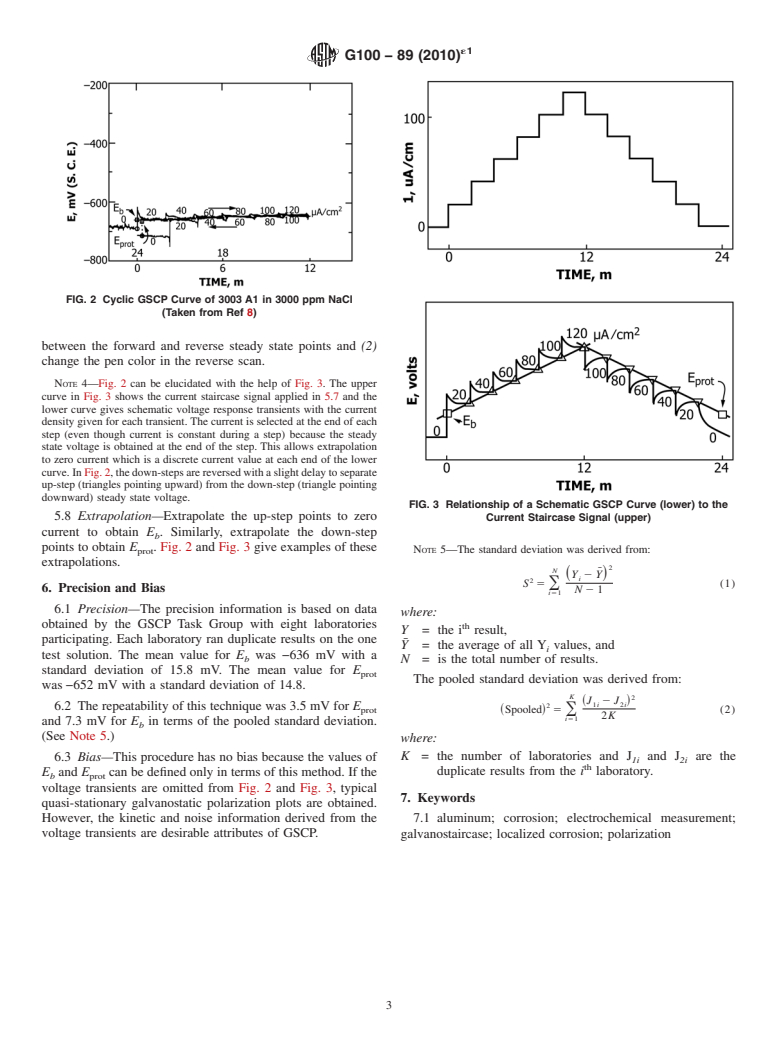
The breakdown (Eb), and protection potentials (Eprot) determined by the cyclic GSCP method correlate with the constant potential corrosion test (immersion-glassware) result for aluminum (1, 6, 8). When the applied potential was more negative than the GSCP Eprot, no pit initiation was observed. When the applied potential was more positive than the GSCP Eprot, pitting occurred even when the applied potential was less negative than Eb.
Severe crevice corrosion occurred when the separation of Eb and Eprot was 500 mV or greater and Eprot was less than −400 mV Vs. SCE (in 100 ppm NaCl) (1, 6, 7). For aluminum, Eprot determined by cyclic GSCP agrees with the repassivation potential determined by the scratch potentiostatic method (1, 10). Both the scratch potentiostatic method and the constant potential technique for determination of Eprot require much longer test times and are more involved techniques than the GSCP method.
DeBerry and Viebeck (3-5) found that the breakdown potentials (Eb) (galvanodynamic polarization, similar to GSCP but no kinetic information) had a good correlation with the inhibition of localized corrosion of 304L stainless steel by surface active compounds. They attained accuracy and precision by avoiding the strong induction effect which they observed by the potentiodynamic technique.
If this test method is followed using the specific alloy discussed it will provide (GSCP) measurements that will reproduce data developed at other times in other laboratories.
Eb and Eprot
obtained are based on the ...
SCOPE
1.1 This test method covers a procedure for conducting cyclic galvanostaircase polarization (GSCP) to determine relative susceptibility to localized corrosion (pitting and crevice corrosion) for aluminum alloy 3003-H14 (UNS A93003) (1). It may serve as guide for examination of other alloys (2-5). This test method also describes a procedure that can be used as a check for one's experimental technique and instrumentation.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: G100 − 89(Reapproved 2010)
Standard Test Method for
Conducting Cyclic Galvanostaircase Polarization
This standard is issued under the fixed designation G100; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Clarified the SI unit statement editorially in May 2010.
1. Scope 3. Significance and Use
3.1 In this test method, susceptibility to localized corrosion
1.1 This test method covers a procedure for conducting
of aluminum is indicated by a protection potential (E )
prot
cyclic galvanostaircase polarization (GSCP) to determine rela-
determined by cyclic galvanostaircase polarization (1). The
tive susceptibility to localized corrosion (pitting and crevice
2 more noble this potential, the less susceptible is the alloy to
corrosion) for aluminum alloy 3003-H14 (UNSA93003) (1).
initiationoflocalizedcorrosion.Theresultsofthistestmethod
It may serve as guide for examination of other alloys (2-5).
are not intended to correlate in a quantitative manner with the
Thistestmethodalsodescribesaprocedurethatcanbeusedas
rate of propagation of localized corrosion that one might
a check for one’s experimental technique and instrumentation.
observe in service.
1.2 The values stated in SI units are to be regarded as
3.2 The breakdown (E ), and protection potentials (E )
b prot
standard. No other units of measurement are included in this
determined by the cyclic GSCP method correlate with the
standard.
constant potential corrosion test (immersion-glassware) result
for aluminum (1, 6, 7). When the applied potential was more
1.3 This standard does not purport to address all of the
negative than the GSCP E , no pit initiation was observed.
safety concerns, if any, associated with its use. It is the
prot
When the applied potential was more positive than the GSCP
responsibility of the user of this standard to establish appro-
E ,pittingoccurredevenwhentheappliedpotentialwasless
priate safety and health practices and determine the applica- prot
negative than E .
bility of regulatory limitations prior to use. b
3.2.1 Severecrevicecorrosionoccurredwhentheseparation
of E and E was 500 mV or greater and E was less
b prot prot
2. Referenced Documents
than−400 mV Vs. SCE (in 100 ppm NaCl) (1, 6, 8). For
2.1 ASTM Standards:
aluminum, E determined by cyclic GSCP agrees with the
prot
D1193Specification for Reagent Water
repassivationpotentialdeterminedbythescratchpotentiostatic
G1Practice for Preparing, Cleaning, and Evaluating Corro-
method (1, 9). Both the scratch potentiostatic method and the
sion Test Specimens
constant potential technique for determination of E require
prot
G5Reference Test Method for Making Potentiodynamic
much longer test times and are more involved techniques than
Anodic Polarization Measurements
the GSCP method.
G59TestMethodforConductingPotentiodynamicPolariza-
3.3 DeBerry and Viebeck (3-5) found that the breakdown
tion Resistance Measurements
potentials (E ) (galvanodynamic polarization, similar to GSCP
b
G69Test Method for Measurement of Corrosion Potentials
but no kinetic information) had a good correlation with the
of Aluminum Alloys
inhibition of localized corrosion of 304L stainless steel by
surface active compounds. They attained accuracy and preci-
sion by avoiding the strong induction effect which they
This test method is under the jurisdiction of ASTM Committee G01 on observed by the potentiodynamic technique.
Corrosion of Metals and is the direct responsibility of Subcommittee G01.11 on
3.4 If this test method is followed using the specific alloy
Electrochemical Measurements in Corrosion Testing.
Current edition approved May 1, 2010. Published May 2010. Originally discussed it will provide (GSCP) measurements that will
approved in 1989. Last previous edition approved in 2004 as G100–89(2004). DOI:
reproduce data developed at other times in other laboratories.
10.1520/G0100-89R10E01.
3.5 E and E obtained are based on the results from eight
The boldface numbers in parentheses refer to a list of references at the end of
b prot
this standard.
different laboratories that followed the standard procedure
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
using aluminum alloy 3003-H14 (UNS A93003). E and E
b prot
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
are included with statistical analysis to indicate the acceptable
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. range.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
G100 − 89 (2010)
(UNSA93003)A1 in sheet form. Cut 1.55 cm diameter circles
and prepare in accordance with Practice G1 using 600-grit
diamond slurry on a flat lapping machine. Install in flat
specimen holder using PTFE gasket (no crevice type) (Note 1)
so that 1 cm is exposed to the test solution.Apply 29 m-g of
torque.
4.3.2 Auxiliary Electrodes—Graphite, (ultrafine grade)
(Note 3).
NOTE 3—Coarse grades of graphite should be avoided because they
absorb solute impurities. Ultrafine grades are available from spectro-
graphic supply companies.
4.3.3 Reference Electrode—Saturated calomel (Note 1). It
should be checked against another reference which has not
been exposed to test solutions and they should be within 3 mV
of each other. Practice G69 round robin test conducted by
G01.11 (unpublished results) indicate that potential difference
should not exceed 2 or 3 mV. The reference electrode is
connected to the test bridge solution which consists of 75%
saturated KCl, prepared by adding 1 part (by volume) of
distilled water to 3 parts saturated KCl. When the bridge is in
active use, the bridge solution should be replaced once each
day and the bridge tip immersed in this solution when not in
use. Any test solution that does not deposit films may also be
usedinthebridge.(TheVYCOR tip should notbeallowedto
go to dryness.)
4.4 Magnetic Stirrer.
FIG. 1 Schematic Wiring Diagram for Galvanostaircase Polariza-
tion
5. Procedure
5.1 Test solution, 3000 6 30 ppm (0.0513 M) NaCl. For
4. Apparatus
example, transfer 6.000 g reagent grade NaCl to a 2-L
volumetric flask. Dissolve in ASTM Type IV water (deminer-
4.1 Cell—The cell should be constructed of inert materials
alized or distilled) and dilute to the mark. (See Specification
such as borosilicate glass and PTFE fluorocarbon. It should
D1193.)
have ports for the insertion of a working electrode (1 cm flat
specimen holder (Note 1) is very convenient), two auxiliary
5.2 Assemble cell with the electrodes described in Section
electrodes, salt bridge for reference electrode, and a thermom-
4.Placethereferencebridgeprobeabout2probetipdiameters
eterorathermostatprobefortemperaturecontrol.Thefigurein
away from the working electrode.
Test Method G5 would be satisfactory, but a flat bottom cell is
5.3 Fill the cell with the test solution so that the level is
also satisfactory provided that all of the essential ports are
about 25 mm above the working electrode.
provided. (See Ref (10) for details.)
5.4 Maintain a temperature of 25 6 1°C.
NOTE 1—These sp
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.