ASTM F1841-97(2013)
(Practice)Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps
Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps
SIGNIFICANCE AND USE
6.1 The objective of this practice is to standardize the evaluation method for detecting the hemolytic effect of a continuous flow blood pump used in extracorporeal circulation and circulatory assistance.
SCOPE
1.1 This practice covers a protocol for the assessment of the hemolytic properties of continuous flow blood pumps used in extracorporeal or implantable circulatory assist. An assessment is made based on the pump's effects on the erythrocytes over a certain period of time. For this assessment, a recirculation test is performed with a pump for 6 h.
1.2 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the two systems may result in non-conformance with the standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F1841 − 97 (Reapproved 2013)
Standard Practice for
Assessment of Hemolysis in Continuous Flow Blood
Pumps
This standard is issued under the fixed designation F1841; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The goal of blood pump development is to replace or supplement the function of the human heart.
As a result, continuous flow blood pumps, including roller pumps and centrifugal pumps, are
commonly used in clinical extracorporeal circulation. They are used not only for cardiopulmonary
bypass in routine cardiac surgery but also for ventricular assist, percutaneous cardiopulmonary
support, and extracorporeal membrane oxygenation.
Many investigators have attempted to develop an atraumatic blood pump. Hemolysis is one of the
most important parameters of blood trauma induced by blood pumps. However, comparative in vitro
evaluation of the reported results of hemolysis are difficult due to the lack of uniformity of the test
methods employed. Thus, it is necessary to standardize the method of performing in vitro hemolysis
tests for the evaluation of continuous flow blood pumps.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This practice covers a protocol for the assessment of the
F1830 PracticeforSelectionofBloodfor in vitroEvaluation
hemolytic properties of continuous flow blood pumps used in
of Blood Pumps
extracorporeal or implantable circulatory assist.An assessment
is made based on the pump’s effects on the erythrocytes over
3. Terminology
acertainperiodoftime.Forthisassessment,arecirculationtest
3.1 Definitions:
is performed with a pump for 6 h.
3.1.1 continuous flow blood pump—a blood pump that
1.2 The values stated in either SI units or inch-pound units
produces continuous blood flow due to its rotary motion.
are to be regarded separately as standard. The values stated in
3.1.2 free plasma hemoglobin—the amount of hemoglobin
each system may not be exact equivalents; therefore, each
(iron or heme-containing protein) in plasma.
system shall be used independently of the other. Combining
3.1.3 hemolysis—damage to erythrocytes resulting in the
values from the two systems may result in non-conformance
liberation of hemoglobin into the plasma.
with the standard.
3.1.4 Index of Hemolysis
1.3 This standard does not purport to address all of the
3.1.4.1 normalized index of hemolysis—added grams of
safety concerns, if any, associated with its use. It is the
plasma free hemoglobin per 100 Lof blood pumped, corrected
responsibility of the user of this standard to establish appro-
for plasma volume using hematocrit and normalized by flow
priate safety and health practices and determine the applica-
rate and circulation time.
bility of regulatory limitations prior to use.
3.1.4.2 normalized milligram index of hemolysis—
normalized index of hemolysis expressed by milligram value
of free plasma hemoglobin.
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.30 onCardiovascular Standards. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved March 1, 2013. Published March 2013. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1997. Last previous edition approved in 2005 as F1841 – 97(2005). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/F1841-97R13. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1841 − 97 (2013)
3.1.4.3 modified index of hemolysis—mass of hemoglobin used in the development stage of a pump, it is suggested that
released into plasma normalized by the total amount of pre-clinical evaluation tests be repeated with human blood.
hemoglobin pumped through the loop.
5. Summary of Practice
4. Formulas
5.1 Blood—The blood is obtained from human volunteers,
4.1 Normalized Index of Hemolysis (N.I.H.) (1, 2, 3, 4) :
cattle or pigs having normal body temperatures, no physical
signs of disease, including diarrhea or rhinorrhea, and an
100 2 Ht 100
N.I.H. g/100l 5∆freeHb 3V 3 3 (1)
acceptable range of hemotological profiles. The blood should
100 Q 3T
be collected by vascular puncture using a needle (14G or
∆free Hb = increase of plasma free hemoglobin concentra-
larger) and collected into the standard 500–2000 mL bags
tion (g/L) over the sampling time interval,
containing citrate phospate dextrose adenine (CPDA-1) anti-
coagulant solution (See Appendix X2) or heparin sulfate (See
where:
Appendix X3). The blood from a slaughterhouse can typically
V = circuit volume (L),
be used if it is obtained by controlled venipuncture.
Q = flow rate (L/min),
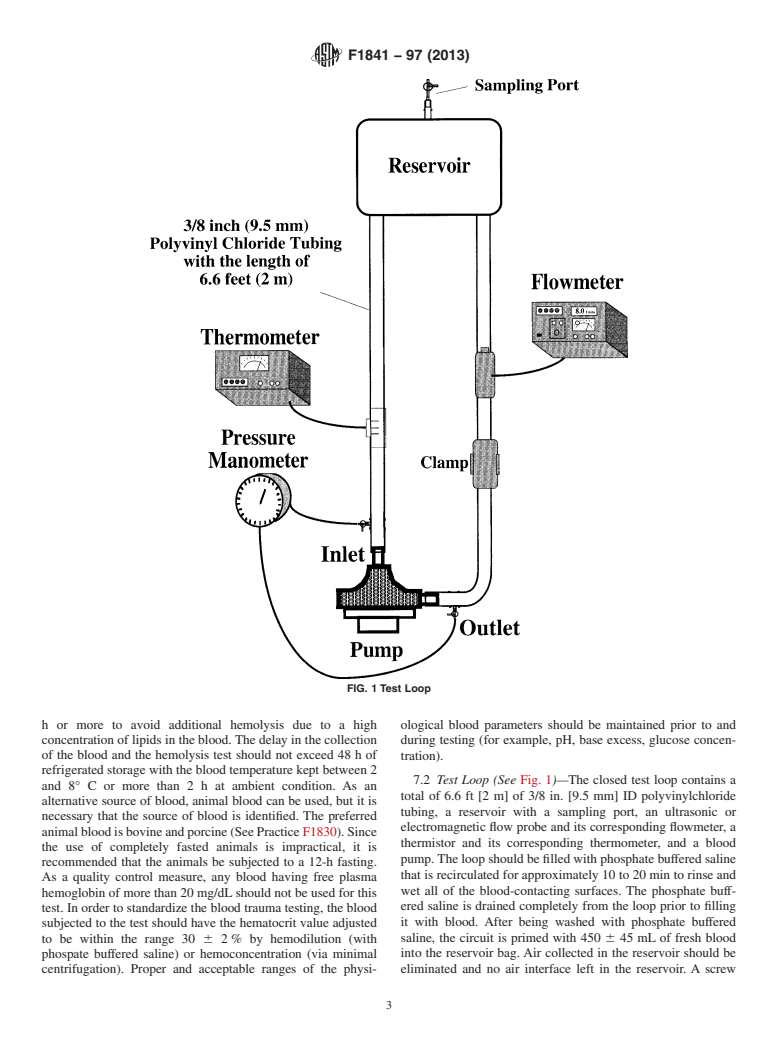
5.2 Test Loop (4) (See Fig. 1)—The test loop consists of a
Ht = hematocrit (%), and
total of 6.6 ft [2 m] of 3/8 in. [9.5 mm] ID polyvinylchloride
T = sampling time interval (min).
tubing and a reservoir (typically, 13 by 13 cm) with a sampling
4.2 Normalized Milligram Index of Hemolysis. (mg.N.I.H.)
port. The primed blood volume is 450 6 45 mL. A screw
(2, 3, 4):
clamp,thatispositionedattheoutletside,isappliedtoproduce
100 2 Ht 100
the required conditions for the left heart assist application (5
2mg.N.I.H.mg/100l 5∆freeHb 3V 3 3 (2)
100 Q 3T
L/min against 100 mm Hg pressure head (that is, with the
pressure sampling ports at the same vertical height, the
4.3 Modified Index of Hemolysis (M.I.H.):
pressure in the outlet line of the pump is 100 mm Hg greater
4.3.1 Modified index of hemolysis (M.I.H.) (5, 6) that can
than in the inlet line)) and for the cardiopulmonary bypass
be written with no units or as (milligram of hemoglobin
application (5 L/min against 500 mm Hg pressure head).
released into plasma/mg of total hemoglobin pumped through
(Optional testing at 350 or 700 mm Hg is also advisable.) To
the loop):
monitor such pressure heads, the pressure monitoring lines are
100 2 Ht 10
M.I.H. 5∆freeHb 3V 3 3 (3) incorporated into the test loop both at the inlet and outlet tubes.
100 Q 3T 3Hb
An ultrasonic or electromagnetic flow probe is placed at the
where:
outlet side of the pump between the clamp and the reservoir to
Hb = total blood hemoglobin concentration at time monitor the flow rate. A thermistor is connected to the loop,
and the blood temperature is measured using a corresponding
zero (mg/L), and
thermometer.
∆free Hb = increase of plasma free hemoglobin concentra-
tion (mg/L) over the sampling time interval.
5.3 Pump Conditions—Pump flow rate is set at 5 6 0.25
L/min at the circulating blood temperature of 37 6 1°C. The
4.3.2 Among these indices, M.I.H. is recommended as an
index to express the degree of hemolysis caused by a blood total pressure head is set at 100 6 3 mm Hg for the left heart
assist application and 500 6 15 mm Hg for cardiopulmonary
pumpinarecirculatingsystem.N.I.H.wasproposedtoaccount
for the plasma volume based on the hemotocrit. Recent bypass application. However, additional testing temperatures
canbechosenfrom0to42°Caccordingtotheintendedclinical
development of less hemolytic blood pumps has since made it
convenient to use mg. N.I.H. rather than N.I.H. However, both use of the pump (for example, cardiopulmonary bypass may
include cooling and warming during surgery.)
the N.I.H. and the mg N.I.H. vary with hematocrit of the blood
(6). M.I.H. is the recommended index to express the degree of
5.4 Evaluation—The free plasma hemoglobin is determined
hemolysis caused by a blood pump in a recirculating system.
by a clinically approved assay method (see 9.3). The free
The M.I.H. equation corrects for differences in blood hemo-
plasma hemoglobin is standardized by calculating the M.I.H.
globin concentration and hematocrit directly (5).
6. Significance and Use
4.4 Testing Blood—Because the level of trauma-induced
hemolysis is different based on the source of blood, it is
6.1 The objective of this practice is to standardize the
necessary to identify the source of blood and its respective
evaluation method for detecting the hemolytic effect of a
index of hemolysis. Human, bovine, or porcine blood are
continuous flow blood pump used in extracorporeal circulation
recommended as the primary sources of testing blood (see
and circulatory assistance.
Practice F1830). It is preferable that the blood collected at a
standard slaughter house not be used due to the risk of being
7. Preparation of Hemolysis Test
contaminated with fluids other than blood, unless the blood is
7.1 Blood—The blood is obtained from human volunteers
obtained by controlled venipuncture.Although animal blood is
having normal body temperature, exhibiting no physical signs
of disease and having hematological profiles in the normal
acceptable range. (Donors are subjected to standard blood
The boldface numbers given in parentheses refer to a list of references at the
end of the text. donor screening procedures.) The donor should be fasted for 8
F1841 − 97 (2013)
FIG. 1 Test Loop
h or more to avoid additional hemolysis due to a high ological blood parameters should be maintained prior to and
concentration of lipids in the blood. The delay in the collection during testing (for example, pH, base excess, glucose concen-
of the blood and the hemolysis test should not exceed 48 h of
tration).
refrigerated storage with the blood temperature kept between 2
7.2 Test Loop (See Fig. 1)—The closed test loop contains a
and 8° C or more than2hat ambient condition. As an
total of 6.6 ft [2 m] of 3/8 in. [9.5 mm] ID polyvinylchloride
alternative source of blood, animal blood can be used, but it is
tubing, a reservoir wit
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.