ASTM F2996-13
(Practice)Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems
Standard Practice for Finite Element Analysis (FEA) of Non-Modular Metallic Orthopaedic Hip Femoral Stems
SIGNIFICANCE AND USE
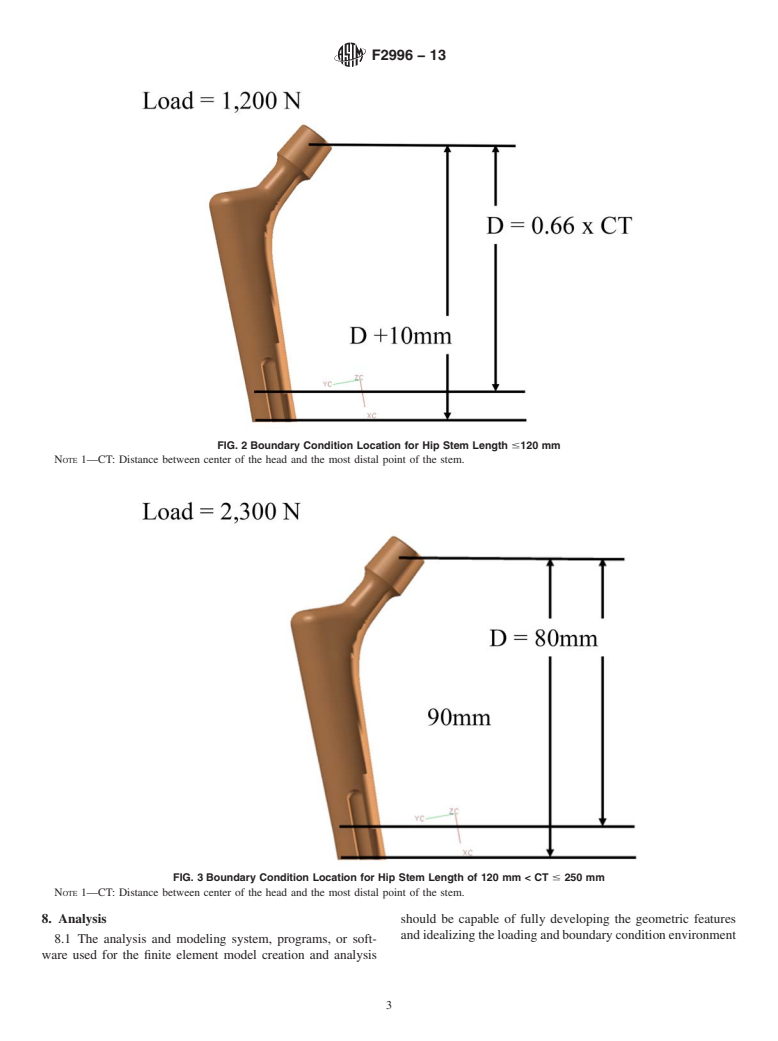
3.1 This practice is applicable to the calculation of stresses seen on a femoral hip stem when loaded in a manner described in ISO 7206-4 (2010). This method can be used to establish the worst case size for a particular implant. When stresses calculated using this practice were compared to the stresses measured from physical strain gauging techniques performed at two laboratories using two different methods, the results correlated to within 8 %.
SCOPE
1.1 This practice establishes requirements and considerations for the numerical simulation of non-modular (that is, limited to monolithic stems with only a femoral head / trunnion taper interface) metallic orthopaedic hip stems using Finite Element Analysis (FEA) techniques for the estimation of stresses and strains. This standard is only applicable to stresses below the yield strength, as provided in the material certification.
1.2 Purpose—This practice establishes requirements and considerations for the development of finite element models to be used in the evaluation of non-modular metallic orthopaedic hip stem designs for the purpose of prediction of the static implant stresses and strains. This procedure can be used for worst case assessment within a family of implant sizes to provide efficiencies in the amount of physical testing to be conducted. Recommended procedures for performing model checks and verification are provided to help determine if the analysis follows recommended guidelines. Finally, the recommended content of an engineering report covering the mechanical simulation is presented.
1.3 Limits—This practice is limited in discussion to the static structural analysis of non-modular metallic orthopaedic hip stems (which excludes the prediction of fatigue strength).
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2996 − 13
Standard Practice for
Finite Element Analysis (FEA) of Non-Modular Metallic
1
Orthopaedic Hip Femoral Stems
This standard is issued under the fixed designation F2996; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
1.1 This practice establishes requirements and consider- 2.1 ISO Standards:
ISO 7206-4 (2010) Implants for Surgery—Partial and Total
ations for the numerical simulation of non-modular (that is,
limitedtomonolithicstemswithonlyafemoralhead/trunnion HipJointProstheses—Part4:DeterminationofEndurance
Properties and Performance of Stemmed Femoral Com-
taper interface) metallic orthopaedic hip stems using Finite
Element Analysis (FEA) techniques for the estimation of ponents
stresses and strains. This standard is only applicable to stresses
3. Significance and Use
below the yield strength, as provided in the material certifica-
3.1 This practice is applicable to the calculation of stresses
tion.
seen on a femoral hip stem when loaded in a manner described
1.2 Purpose—This practice establishes requirements and
in ISO 7206-4 (2010).This method can be used to establish the
considerations for the development of finite element models to
worst case size for a particular implant. When stresses calcu-
be used in the evaluation of non-modular metallic orthopaedic
lated using this practice were compared to the stresses mea-
hip stem designs for the purpose of prediction of the static
sured from physical strain gauging techniques performed at
implant stresses and strains. This procedure can be used for
two laboratories using two different methods, the results
worst case assessment within a family of implant sizes to
correlated to within 8 %.
provide efficiencies in the amount of physical testing to be
conducted. Recommended procedures for performing model
4. Geometric Data
checks and verification are provided to help determine if the
4.1 Finite element models are based on a geometric repre-
analysis follows recommended guidelines. Finally, the recom-
sentation of the device being studied. The source of the
mendedcontentofanengineeringreportcoveringthemechani-
geometricdetailscanbeobtainedfromdrawings,solidmodels,
cal simulation is presented.
preliminary sketches, or any other source consistent with
1.3 Limits—This practice is limited in discussion to the
defining the model geometry. In building the finite element
static structural analysis of non-modular metallic orthopaedic
model, certain geometric details may be omitted from the
hip stems (which excludes the prediction of fatigue strength).
orthopaedic implant geometry shown in the CAD model if it is
determined that they are not relevant to the intended analysis.
1.4 The values stated in SI units are to be regarded as
Engineering judgment shall be exercised to establish the extent
standard. No other units of measurement are included in this
of model simplification and shall be justified.
standard.
4.2 It is most appropriate to consider the “worst case” stress
1.5 This standard does not purport to address all of the
condition for the orthopaedic implant being simulated. The
safety concerns, if any, associated with its use. It is the
“worst case” shall be determined from all relevant engineering
responsibility of the user of this standard to establish appro-
considerations, such as stem geometry, dimensions, and head
priate safety and health practices and determine the applica-
offset. If finite element analysis is being used for determining
bility of regulatory limitations prior to use.
the worst case, then the worst case head offset may not be
known. It may be necessary to run several variants of head
1
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
offset, in order to determine this.
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.22 on Arthroplasty.
2
Current edition approved July 15, 2013. Published August 2013. DOI: 10.1520/ Available from International Organization for Standardization (ISO), 1, ch. de
F2996-13. la Voie-Creuse, CP 56, CH-1211 Geneva 20, Switzerland, http://www.iso.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2996 − 13
5. Material Properties to approximate the loading conditions that simulate the worst
case head offset, which may be determined via an iterative
5.1 The required material properties for input into an FEA
process. This approximation should be reported if performed.
model for the calc
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.