ASTM F2394-04
(Guide)Standard Guide for Measuring Securement of Balloon Expandable Stent Mounted on Delivery System
Standard Guide for Measuring Securement of Balloon Expandable Stent Mounted on Delivery System
SCOPE
1.1 This guide provides guidance for the design and development of pre-test treatments, tests, and test endpoints to measure stent securement of pre-mounted, unsheathed, balloon-expandable stent delivery systems. This guide is intended to aid investigators in the design, development, and in vitro characterization of pre-mounted, unsheathed, balloon-expandable stent delivery systems.
1.2 This guide covers the laboratory determination of the shear force required to displace or dislodge a balloon-expandable endovascular stent mounted on a delivery system. The guide proposes a set of options to consider when testing stent securement. The options cover pre-test treatments, possible stent securement tests, and relevant test endpoints. An example test apparatus is given in 7.1.
1.3 This guide covers in vitro bench testing characterization only. Measured levels of securement and product design/process differentiation may be particularly influenced by selections of pre-test treatments, securement test type (for example, stent gripping method), and test endpoint. In vivo characteristics may also differ from in vitro results.
1.4 This guide does not cover all possible pre-test treatments, stent securement tests, or test endpoints. It is intended to provide a starting point from which to select and investigate securement test options.
1.5 This guide does not specify a method for mounting the stent onto the delivery system.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory requirements prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F2394–04
Standard Guide for
Measuring Securement of Balloon Expandable Stent
Mounted on Delivery System
This standard is issued under the fixed designation F 2394; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope E 1488 Guide for Statistical Procedures to use in Develop-
ing and Applying Test Methods
1.1 This guide provides guidance for the design and devel-
2.2 Other Documents:
opment of pre-test treatments, tests, and test endpoints to
ISO 10555-1 Sterile Sterile, Single-use Intravascular
measure stent securement of pre-mounted, unsheathed,
Catheters—Part 1: General Requirements
balloon-expandable stent delivery systems. This guide is in-
Quality System Regulation, Part VII Dept. Health and
tended to aid investigators in the design, development, and in
Human Services, Food and DrugAdministration, 21 CFR
vitro characterization of pre-mounted, unsheathed, balloon-
Part 820 Medical Devices; Current Good Manufacturing
expandable stent delivery systems.
Practice; Final Rule. Federal Register, October 7, 1996
1.2 This guide covers the laboratory determination of the
shear force required to displace or dislodge a balloon-
3. Terminology
expandable endovascular stent mounted on a delivery system.
3.1 Definitions:
The guide proposes a set of options to consider when testing
3.1.1 balloon expandable stent, n—a stent that is expanded
stent securement. The options cover pre-test treatments, pos-
at the treatment site by a balloon catheter. The stent material is
sible stent securement tests, and relevant test endpoints. An
plastically deformed by the balloon expansion such that the
example test apparatus is given in 7.1.
stent remains expanded after deflation of the balloon.
1.3 This guide covers in vitro bench testing characterization
3.1.2 crimp, v—tosecurethestentonthedeliverysystemby
only. Measured levels of securement and product design/
radially compressing and plastically deforming the stent onto
process differentiation may be particularly influenced by selec-
the balloon.
tions of pre-test treatments, securement test type (for example,
3.1.3 delivery system, n—a system similar to a balloon
stent gripping method), and test endpoint. In vivo characteris-
dilatation catheter that is used to deliver and deploy a stent at
tics may also differ from in vitro results.
the target site.
1.4 This guide does not cover all possible pre-test treat-
3.1.4 displacement force, critical distance peak, n—a stent
ments,stentsecurementtests,ortestendpoints.Itisintendedto
securement test endpoint characterizing the maximum force
provide a starting point from which to select and investigate
required to displace the stent with respect to the balloon a
securement test options.
critical distance. This critical distance is the minimum of the
1.5 This guide does not specify a method for mounting the
following two distances. The first is the distance at which the
stent onto the delivery system.
undamaged stent could overhang the balloon body resulting in
1.6 This standard does not purport to address all of the
a clinically significant, incomplete end deployment. The sec-
safety concerns, if any, associated with its use. It is the
ond is the length (distance) of stent compression or buckling
responsibility of the user of this standard to establish appro-
that could result in a clinically significant, incomplete deploy-
priate safety and health practices and determine the applica-
ment of the stent against the vessel walls. (See Fig. X2.1.)
bility of regulatory requirements prior to use.
3.1.5 displacement force, initial, n—a stent securement test
2. Referenced Documents endpointcharacterizingtheinitialforcerequiredtodisplacethe
2 stent with respect to the balloon such that the displacement is
2.1 ASTM Standards:
a non-recoverable movement (see definition below). (See Fig.
X2.1.)
This guide is under the jurisdiction of ASTM Committee F04 on Medical and
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.30 on Cardiovascular Standards.
Current edition approved May 1, 2004. Published June 2004.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM 4th Floor, New York, NY 10036.
Standards volume information, refer to the standard’s Document Summary page on AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
the ASTM website. 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2394–04
3.1.6 displacement force, initial peak, n—a stent secure- engineering diagrams simulate vessels with a moderately
ment test endpoint characterizing the first peak in force that difficult degree of coronary tortuousity but do not include
simulated lesions.
occurs during or after stent displacement with respect to the
3.1.17 pre-test treatment lesion fixture, n—a pre-test treat-
balloon. (See Fig. X2.1.)
ment fixture used to simulate an anatomical vasculature and
3.1.7 dislodgment force, peak, n—a stent securement test
lesion. Use of the fixture with a guide catheter, a guide wire,
endpointcharacterizingthepeakormaximumforcerequiredto
and the stent-balloon catheter delivery system is intended to
completelydislodgethestentfromthedeliverysystemballoon.
simulate the bending, frictional and mechanical resistance
During a test, this force will occur after or coincide with the
forces of tracking the device across the lesion site that may be
initial displacement force. (See Fig. X2.1.)
encountered in the clinical setting.
3.1.8 failure mode effect analysis (FMEA), n—an analytical
3.1.18 securement test, guide-type, n—a stent securement
approach to methodically determine and address all possible
test that is similar to the clinical scenario of pulling an
product failure modes, their associated causes, and their
undeployed stent delivery system back into a guide catheter,
criticality. Used to evaluate designs, prioritize testing, and
arterial sheath or hemostasis valve. Examples include guides,
track risk reducing improvements to the product.
rings,orshimsideallydesignedtoengagethestentendorbody
3.1.9 gage length, n—the initial unstressed length of cath-
but not the catheter balloon. The shim securement test, de-
eter tubing between the proximal end of the stent to the grips
scribedinSection7,usescomplementarythin,rigidplateswith
which engage the catheter tubing. rounded “V” notches that are sized to circumferentially engage
the stent end but not the catheter balloon. See the engineering
3.1.10 grips, n—a means of applying force to the stent and
diagrams in the Appendix.
balloon catheter to displace or dislodge the stent relative to the
3.1.19 securement test, lesion-type, n—a stent securement
balloon. In particular, grips refer to the end of a device which
test that is similar to the clinical scenario of pushing or pulling
makes the contact with the stent. Typical grips used to apply
an undeployed stent delivery system through or around a
force to the stent include shims (as used in Figs. X2.5-X2.8);
fibrous or calcified lesion. Examples include tape, nubs,
tape which sticks to the stent but not the balloon; an iris which
protrusions or sandpaper ideally designed to engage the stent
can be narrowed down to allow the balloon to slip by but not
end or body but not the catheter balloon.
the stent; or nubs which contact the stent but not the balloon.
3.1.11 guide catheter, n—a tube designed to transport the
4. Significance and Use
guidewire and the stent delivery system into the target vessel.
4.1 The securement of the endovascular stent on the balloon
3.1.12 guidewire, n—a wire designed to aid in balloon,
isacriticalparametertoensurethatthestentissafelydelivered
ultrasound, atherectomy, or stent placement during endovascu-
to or from the treatment site.
lar procedures.
4.2 This guide is intended for use by researchers and
3.1.13 mandrel, n—awirethatmaybeusedasanalternative
manufacturers for the selection of pre-test treatments, tests and
to the intended guide wire to provide support for the catheter test endpoints to measure stent securement (displacement
guide wire lumen for some test procedures. and/or dislodgment forces).
4.3 This guide may be used to investigate which practical
3.1.14 non-recoverable movement, n—adisplacementofthe
combinations of in vitro tests best characterize clinical sce-
stent relative to the balloon such that if the shearing force was
narios.
reduced to zero, the stent would remain displaced in the
4.4 This guide should be used with discretion in choosing
direction of the shearing force relative to the initial placement
securement tests and evaluating results due to the myriad
on the balloon. The force at which non-recoverable movement
possible combinations of clinical conditions, failure modes,
begins is defined as the initial displacement force (see defini-
and stent delivery system designs.
tion above).
3.1.15 pre-test treatment, n—a treatment of the stent deliv-
5. Clinical Scenarios
ery system prior to the evaluation of securement that simulates
5.1 There are two failure modes—the stent is dislodged
preparatory, environmental, mechanical or other conditions
from the catheter or the stent is displaced or deformed on the
that may be encountered prior to or during clinical use of the
catheter such that balloon inflation delivery would not produce
device. Examples include subjecting the devices to elevated
an acceptable stent shape at the proper location. Based on
shipping temperature/humidity, catheter preparation per use
reported clinical incidents, there are three causes for these two
instructions, pre-soaking, bending treatments, tracking treat-
types of failures:
ments (tracking fixture, see definition below) and tracking
5.1.1 Displacement or dislodgment of the stent while at-
through lesion treatments (lesion fixture, see definition below).
tempting to track through or position in tortuous bends, fibrous
3.1.16 pre-test treatment tracking fixture, n—a pre-test
or calcified lesions, or previously implanted stents, or combi-
treatment fixture used to simulate an anatomical vasculature.
nation thereof.
Use of the fixture with a guide catheter, a guide wire and the
5.1.2 Displacement or dislodgment of the stent on with-
stent-balloon catheter delivery system is intended to simulate
drawal of the undeployed stent delivery system back into the
the bending and frictional forces of tracking the device to the guide catheter, introducer sheath, or hemostasis valve. This
lesion site that may be encountered in the clinical setting. See
failure type is usually associated with failure to cross tortuous
the engineering diagrams in the Appendix. Note that these bends, fibrous or calcified lesions, or previously implanted
F2394–04
stents, or combination thereof. It is sometimes associated with observed prior to use but may conceivably play a role in a
less-than-ideal seating or angled placement of the guide small percentage of cases where dislodgment occurs in pa-
catheter tip in the ostium of the vessel. tients.
5.1.3 Displacement or dislodgement of the stent due to
6. Test Method Considerations
improper catheter preparation including mishandling or partial
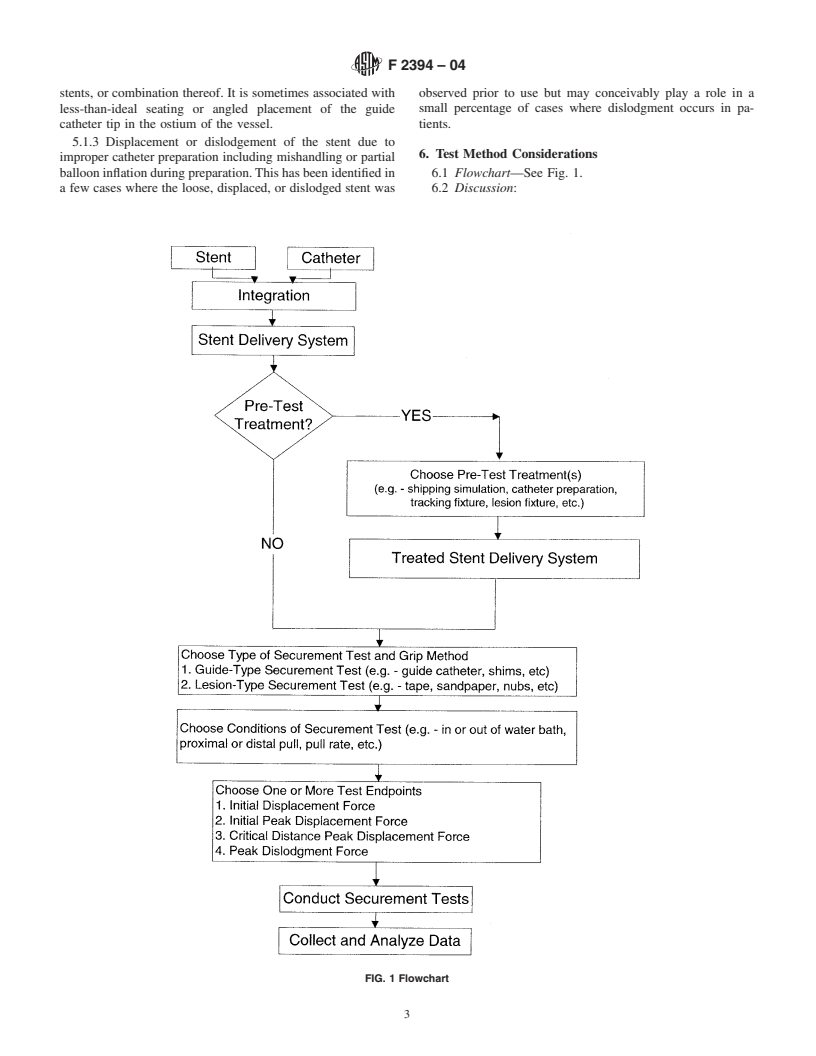
ballooninflationduringpreparation.Thishasbeenidentifiedin 6.1 Flowchart—See Fig. 1.
a few cases where the loose, displaced, or dislodged stent was 6.2 Discussion:
FIG. 1 Flowchart
F2394–04
6.2.1 Securement test development and selection is ideally els. An example of a tracking treatment is given in Section 7.
begun through the initial use of a battery of tests measuring a Two examples of tracking fixtures are given in engineering
variety of failure modes. These test methods may vary from a diagrams in the Appendix. Note that these engineering dia-
simple intuitive tactile impression of the securement forces grams simulate vessels in 2-D with a moderately difficult
through manipulation to clinically modeled situations with degree of coronary tortuousity but do not include simulated
guide catheters and stenosis models to in vivo animal studies lesions.
with representative anatomy and physician handling. 6.3.4 Tracking through lesion treatments are intended to
clinically simulate the bending, frictional and mechanical
6.2.2 The final securement test(s) selected must ultimately
resistance forces of tracking the device across the lesion site.
satisfy internal manufacturer quality standards. These stan-
Considerations include those of tracking treatments in addition
dards may include clinical relevance, FMEA analysis, statisti-
tosimulatingthelesionmaterial,type,andmorphology.Lesion
cal assurance of characteristics, and challenge assurance of
material encountered clinically may include calcium, fibrin,
characteristics.
collagen, fat, cholesterol, endothelial cells, smooth muscle
6.2.3 The final securement test(s) must also satisfy external
cells, red blood cells, platelets, dead white blood cells, and
regulatorybodystandards.Forexample,theFDAQSR21CFR
macrophages. Lesion types may be calcified, fibrous, lipidic,
Part 820, Oct. 7, 1996 states that each test used in the process
thrombotic, or grumous. Lesion morphologies may be totally
ofdesignandmanufacturingoffinisheddevices“issuitablefor
or partially occluded, concentric or eccentric, focal or diffuse,
its intended purposes and is capable of producing valid
and of many possible shapes.
results.” For the statistical capability, Guide E 1488 provides
6.4 Securement Test and Grip Methods:
guidance.
6.4.1 There are two main categories of stent securement
6.2.4 Thisguideoutlinesthepathfrominitialtestingtofinal
tests: guide-types and lesion-types.
test method selection to provide assurance of stent securement
6.4.2 A guide-type securement test simulates the clinical
characteristics.
scenario of pulling an undeployed stent delivery system back
6.2.5 The validation activities for a test method depend on
into a guide catheter, arterial sheath or hemostasis valve.
the criticality and the complexity of the test method. That is,
Examples of grip methods used to simulate guide-type secure-
the more complex the test, the more work will be required to
ment tests include guide catheters, rings, or shims ideally
understand the method, sources of variation (test
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.