ASTM F2559/F2559M-21
(Guide)Standard Guide for Writing a Specification for Sterilizable Peel Pouches
Standard Guide for Writing a Specification for Sterilizable Peel Pouches
SIGNIFICANCE AND USE
5.1 Medical device peel pouches are universally used by the industry and produced by a myriad of suppliers. They may be constructed of many different materials including films, foils, paper, nonwovens such as Tyvek, and combinations thereof. However, even with the diversity of materials, there are still basic requirements that all pouches should exhibit. Above all, the pouches must contain and protect the device while maintaining sterility during all physical handling.
5.2 Pouch requirements may be divided into two categories, initial pouch and material qualification, and routine production and receipt requirements to ensure the purchaser receives exactly what is ordered. While all requirements should be included in the written specification, initial qualification tests may only be needed prior to the first order. Routine production and receipt requirements should be adhered to on every order. Initial qualification requirements are indicated within each clause, where applicable.
5.3 This guide provides an understanding of the requirements needed for the manufacture, purchase, and acceptance of a preformed peelable pouch. Appropriate test methods for compliance are also cited.
Note 1: All test methods for a particular requirement may not be cited due to specific or unique circumstances. For additional guidance on applicable methods, refer to Guide F2097.
5.4 The specification and its requirements should be mutually agreed to by the supplier and purchaser of pouches. This helps ensure that pouches will comply to specified requirements.
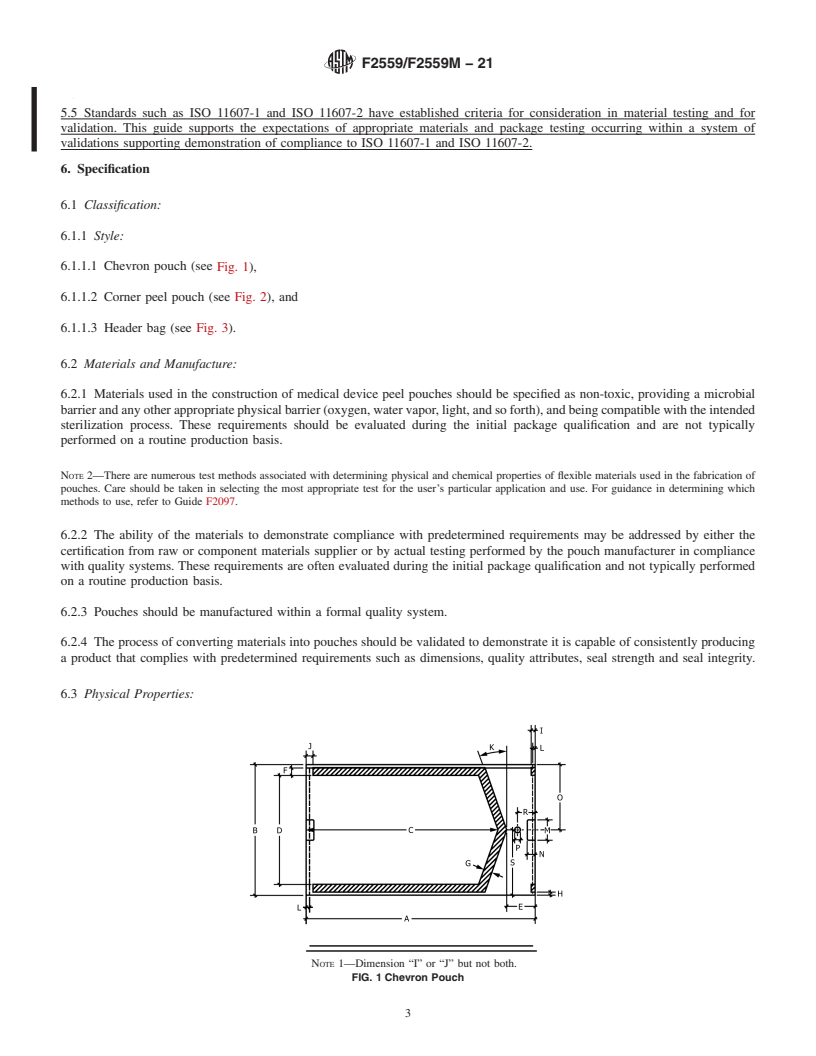
5.5 Standards such as ISO 11607-1 and ISO 11607-2 have established criteria for consideration in material testing and for validation. This guide supports the expectations of appropriate materials and package testing occurring within a system of validations supporting demonstration of compliance to ISO 11607-1 and ISO 11607-2.
SCOPE
1.1 This guide defines the requirements and considerations for flexible peel pouches with one open, unsealed end that are intended to be sterilized containing medical devices. These are also known as preformed sterile barrier systems.
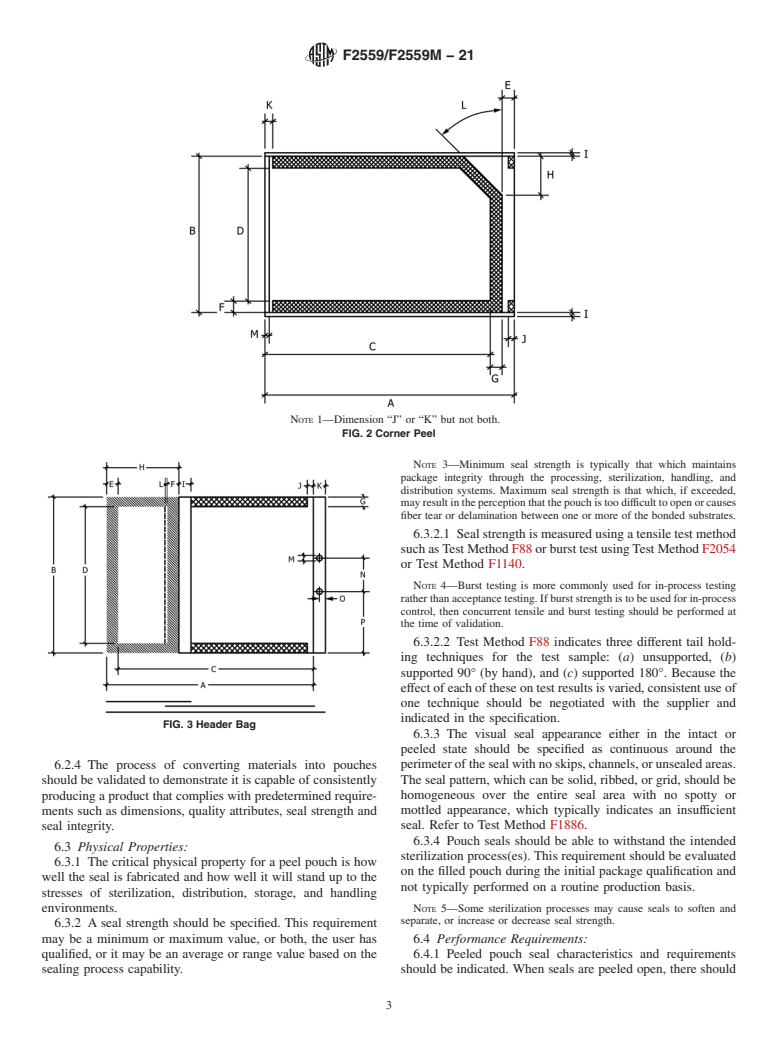
1.2 Pouch styles are categorized as chevron, header, and corner peel. These pouches are typically manufactured by heat sealing, or in some cases, by cohesive cold sealing. The sealing bond is intended to be peeled open to aseptically dispense the contents.
1.3 Pouch materials may be either porous, nonporous, or any combination of the two.
1.4 This guide addresses some critical printing requirements on the pouch.
1.5 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the two systems may result in non-conformance with the standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation:F2559/F2559M −21
Standard Guide for
1
Writing a Specification for Sterilizable Peel Pouches
ThisstandardisissuedunderthefixeddesignationF2559/F2559M;thenumberimmediatelyfollowingthedesignationindicatestheyear
of original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval.
A superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
2.1 ASTM Standards:
1.1 This guide defines the requirements and considerations
E122 Practice for Calculating Sample Size to Estimate,With
for flexible peel pouches with one open, unsealed end that are
Specified Precision, the Average for a Characteristic of a
intended to be sterilized containing medical devices. These are
Lot or Process
also known as preformed sterile barrier systems.
F17 Terminology Relating to Primary Barrier Packaging
1.2 Pouch styles are categorized as chevron, header, and
F88 Test Method for Seal Strength of Flexible Barrier
corner peel. These pouches are typically manufactured by heat
Materials
sealing, or in some cases, by cohesive cold sealing.The sealing
F1140 Test Methods for Internal Pressurization Failure Re-
bond is intended to be peeled open to aseptically dispense the
sistance of Unrestrained Packages
contents.
F1886 Test Method for Determining Integrity of Seals for
1.3 Pouch materials may be either porous, nonporous, or Flexible Packaging by Visual Inspection
any combination of the two.
F2054 Test Method for Burst Testing of Flexible Package
Seals Using InternalAir PressurizationWithin Restraining
1.4 Thisguideaddressessomecriticalprintingrequirements
Plates
on the pouch.
F2097 Guide for Design and Evaluation of Primary Flexible
1.5 The values stated in either SI units or inch-pound units
Packaging for Medical Products
are to be regarded separately as standard. The values stated in
F2203 TestMethodforLinearMeasurementUsingPrecision
each system may not be exact equivalents; therefore, each
Steel Rule
system shall be used independently of the other. Combining
F2250 Practice for Evaluation of Chemical Resistance of
values from the two systems may result in non-conformance
Printed Inks and Coatings on Flexible Packaging Materi-
with the standard.
als
F2475 Guide for Biocompatibility Evaluation of Medical
1.6 This standard does not purport to address all of the
Device Packaging Materials
safety concerns, if any, associated with its use. It is the
3
2.2 TAPPI Standards:
responsibility of the user of this standard to establish appro-
T213 Dirt in Pulp – Chart Metho
priate safety, health, and environmental practices and deter-
T437 Dirt in Paper and Paperboard
mine the applicability of regulatory limitations prior to use.
T564 Transparent Chart for the Estimation of Defect Size
1.7 This international standard was developed in accor-
4
2.3 ISO Standards:
dance with internationally recognized principles on standard-
ISO 11607-1 Packaging for terminally sterilized medical
ization established in the Decision on Principles for the
devices — Part 1: Requirements for materials, sterile
Development of International Standards, Guides and Recom-
barrier systems, and packaging systems
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
1
This guide is under the jurisdiction of ASTM Committee F02 on Primary the ASTM website.
3
Barrier Packaging and is the direct responsibility of Subcommittee F02.50 on Available from Technical Association of the Pulp and Paper Industry (TAPPI),
Package Design and Development. 15 Technology Parkway South, Norcross, GA 30092, http://www.tappi.org.
4
Current edition approved Oct. 1, 2021. Published November 2021. Originally Available from International Organization for Standardization (ISO), ISO
approved in 2006. Last previous edition approved in 2015 as F2559/F2559M – 06 Central Secretariat, Chemin de Blandonnet 8, CP 401, 1214 Vernier, Geneva,
(2015). DOI: 10.1520/F2559_F2559M-21. Switzerland, https://www.iso.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2559/F2559M−21
ISO 11607-2 Packaging for terminally sterilized medical 5.4 The specification and its requirements should be mutu-
devices — Part 2: Validation requirements for forming, ally agreed to by the supplier and pu
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2559/F2559M − 06 (Reapproved 2015) F2559/F2559M − 21
Standard Guide for
1
Writing a Specification for Sterilizable Peel Pouches
This standard is issued under the fixed designation F2559/F2559M; the number immediately following the designation indicates the year
of original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval.
A superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide defines the requirements and considerations for flexible peel pouches with one open, unsealed end that are intended
to be sterilized containing medical devices. These are also known as preformed sterile barrier systems.
1.2 Pouch styles are categorized as chevron, header, and corner peel. These pouches are typically manufactured by heat sealing,
or in some cases, by cohesive cold sealing. The sealing bond is intended to be peeled open to aseptically dispense the contents.
1.3 Pouch materials may be either porous, nonporous, or any combination of the two.
1.4 This guide addresses some critical printing requirements on the pouch.
1.5 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each
system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the
two systems may result in non-conformance with the standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E122 Practice for Calculating Sample Size to Estimate, With Specified Precision, the Average for a Characteristic of a Lot or
Process
F17 Terminology Relating to Primary Barrier Packaging
F88 Test Method for Seal Strength of Flexible Barrier Materials
F1140 Test Methods for Internal Pressurization Failure Resistance of Unrestrained Packages
F1886 Test Method for Determining Integrity of Seals for Flexible Packaging by Visual Inspection
F2054 Test Method for Burst Testing of Flexible Package Seals Using Internal Air Pressurization Within Restraining Plates
1
This guide is under the jurisdiction of ASTM Committee F02 on FlexiblePrimary Barrier Packaging and is the direct responsibility of Subcommittee F02.50 on Package
Design and Development.
Current edition approved Oct. 1, 2015Oct. 1, 2021. Published October 2015November 2021. Originally approved in 2006. Last previous edition approved in 20102015
ϵ1
as F2559/F2559M-06(2010)F2559/F2559M . DOI: 10.1520/F2559_F2559M-06R15. – 06 (2015). DOI: 10.1520/F2559_F2559M-21.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2559/F2559M − 21
F2097 Guide for Design and Evaluation of Primary Flexible Packaging for Medical Products
F2203 Test Method for Linear Measurement Using Precision Steel Rule
F2250 Practice for Evaluation of Chemical Resistance of Printed Inks and Coatings on Flexible Packaging Materials
F2475 Guide for Biocompatibility Evaluation of Medical Device Packaging Materials
3
2.2 TAPPI Standards:
T213 Dirt in Pulp – Chart Metho
T437 Dirt in Paper and Paperboard
T564 Transparent Chart for the Estimation of Defect Size
4
2.3 ISO Standards:
ISO 11607-1 Packaging for terminally sterilized medical devices — Part 1: Requirements for materials, sterile barrier systems,
and packaging systems
ISO 11607-2 Packaging for terminally sterilized medical devices — Part 2: Validation requirements for forming, sealing, and
assembly processes
3. Terminology
3.1 Definitions—For definitions and terms used in this guide, see Terminology F17.
3.2 Definitio
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.