ASTM E1558-09(2021)
(Guide)Standard Guide for Electrolytic Polishing of Metallographic Specimens
Standard Guide for Electrolytic Polishing of Metallographic Specimens
SIGNIFICANCE AND USE
4.1 Advantages of Electrolytic Polishing:
4.1.1 For some metals, a high quality surface finish can be produced that is equivalent to, or better than, that which can be obtained by mechanical methods.
4.1.2 Once procedures have been established, satisfactory results can be obtained rapidly with reproducibility.
4.1.3 There can be a marked saving of time if many specimens of the same material are polished sequentially.
4.1.4 Electropolishing a selected area on the surface of a relatively large metal part can be accomplished nondestructively, that is, without the need for sectioning to remove a piece.
4.1.5 Soft, single-phase metals, which may be difficult to polish by mechanical methods, may be successfully electropolished.
4.1.6 The true microstructure of a specimen can be obtained because artifacts (such as disturbed metal, scratches, and mechanical twins) produced on the surface, even by careful grinding and mechanical polishing operations, can be removed. These features are important in low-load hardness testing, X-ray diffraction studies, and in electron microscopy, where higher resolution puts a premium on undistorted metal surfaces.
4.1.7 After electropolishing is completed, etching can often be accomplished by reducing the voltage (generally to about one-tenth that required for polishing) for a short time before it is turned off.
Note 2: Not all electropolishing solutions produce good etching results.
4.2 Disadvantages of Electrolytic Polishing:
4.2.1 Many of the chemical mixtures used in electropolishing are poisonous or dangerous if not properly handled (see Section 5). These hazards are similar to those involved in the mixing and handling of etchants, see Test Methods E407.
4.2.2 In multi-phase alloys, the polishing rate of each phase may be different. The result may be a non-planar surface.
4.2.3 Electropolished surfaces may be slightly undulated rather than perfectly planar and, therefore, may not be suitable for exami...
SCOPE
1.1 This guide deals with electrolytic polishing as a means of preparation of specimens for metallographic purposes. Procedures are described for polishing a variety of metals.
Note 1: References (1-133)2 on electrolytic polishing will provide the reader with specific information beyond the scope of this guide.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are described in Section 5 and 6.3.1.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E1558 − 09 (Reapproved 2021)
Standard Guide for
Electrolytic Polishing of Metallographic Specimens
This standard is issued under the fixed designation E1558; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2.1 electrolytic polish (electropolish)—A method of pol-
ishingmetalsandalloysinwhichmaterialisremovedfromthe
1.1 This guide deals with electrolytic polishing as a means
surface by making the metal the anode in an electrolytic bath.
of preparation of specimens for metallographic purposes.
Procedures are described for polishing a variety of metals.
4. Significance and Use
NOTE 1—References (1-133) on electrolytic polishing will provide the
4.1 Advantages of Electrolytic Polishing:
reader with specific information beyond the scope of this guide.
4.1.1 For some metals, a high quality surface finish can be
1.2 The values stated in SI units are to be regarded as producedthatisequivalentto,orbetterthan,thatwhichcanbe
standard. No other units of measurement are included in this obtained by mechanical methods.
standard. 4.1.2 Once procedures have been established, satisfactory
results can be obtained rapidly with reproducibility.
1.3 This standard does not purport to address all of the
4.1.3 There can be a marked saving of time if many
safety concerns, if any, associated with its use. It is the
specimens of the same material are polished sequentially.
responsibility of the user of this standard to establish appro-
4.1.4 Electropolishing a selected area on the surface of a
priate safety, health, and environmental practices and deter-
relatively large metal part can be accomplished
mine the applicability of regulatory limitations prior to use.
nondestructively, that is, without the need for sectioning to
Specific safety precautions are described in Section 5 and
remove a piece.
6.3.1.
4.1.5 Soft, single-phase metals, which may be difficult to
1.4 This international standard was developed in accor-
polish by mechanical methods, may be successfully electrop-
dance with internationally recognized principles on standard-
olished.
ization established in the Decision on Principles for the
4.1.6 Thetruemicrostructureofaspecimencanbeobtained
Development of International Standards, Guides and Recom-
because artifacts (such as disturbed metal, scratches, and
mendations issued by the World Trade Organization Technical
mechanical twins) produced on the surface, even by careful
Barriers to Trade (TBT) Committee.
grindingandmechanicalpolishingoperations,canberemoved.
These features are important in low-load hardness testing,
2. Referenced Documents
X-ray diffraction studies, and in electron microscopy, where
2.1 ASTM Standards:
higher resolution puts a premium on undistorted metal sur-
E7Terminology Relating to Metallography
faces.
E407Practice for Microetching Metals and Alloys
4.1.7 After electropolishing is completed, etching can often
be accomplished by reducing the voltage (generally to about
3. Terminology
one-tenth that required for polishing) for a short time before it
3.1 Definitions—All terms used in this guide are either
is turned off.
defined in Terminology E7 or are discussed in 3.2.
NOTE 2—Not all electropolishing solutions produce good etching
3.2 Definitions of Terms Specific to This Standard:
results.
4.2 Disadvantages of Electrolytic Polishing:
1 4.2.1 Many of the chemical mixtures used in electropolish-
ThisguideisunderthejurisdictionofASTMCommitteeE04onMetallography
and is the direct responsibility of Subcommittee E04.01 on Specimen Preparation.
ing are poisonous or dangerous if not properly handled (see
Current edition approved Sept. 1, 2021. Published November 2021. Originally
Section 5). These hazards are similar to those involved in the
approved in 1993. Last previous edition approved in 2014 as E1558–09(2014).
mixing and handling of etchants, see Test Methods E407.
DOI: 10.1520/E1558-09R21.
4.2.2 In multi-phase alloys, the polishing rate of each phase
The boldface numbers in parentheses refer to the references at the end of this
standard.
may be different. The result may be a non-planar surface.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.2.3 Electropolished surfaces may be slightly undulated
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
rather than perfectly planar and, therefore, may not be suitable
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. for examination at all magnifications.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1558 − 09 (2021)
4.2.4 The rate of polishing in areas adjacent to various must only be used in an exclusive hood equipped with a wash
inhomogeneities, such as nonmetallic inclusions and voids, is down feature. To avoid the accumulation of explosive, dry
usuallygreaterthanthatinthesurroundingmatrixandtendsto perchlorates, the hood should undergo a wash down cycle
exaggerate the size of the inclusions and voids. following each use.
4.2.5 Dimples, pits, and waviness limit applications involv-
5.2.2 When pouring, mixing, or using electrolytes, always
ingsurfacephenomena,coatings,interfaces,andcracks.Edges
use the proper protective equipment (eyewear, gloves, apron,
tend to be attacked preferentially, resulting in edge rounding.
and so forth).
4.2.6 Artifacts may be produced by electropolishing.
5.2.3 Use proper devices (glass or plastic) for weighing,
4.2.7 Specimen mounting materials may react with the
measuring, mixing, containing, and storage of solutions.
electrolyte.
5.2.4 When mixing electrolytes, always add reagents to the
4.2.8 The electropolished surfaces of certain materials may
solvent unless specific instructions indicate otherwise.
be passive and difficult to etch.
5.2.5 When using an electrolyte, always avoid direct physi-
4.2.9 Metal removal rates by electropolishing are usually
cal contact with the electrolyte and the specimen. Use tongs or
quite low, typically about 1 µm/min, and all of the prior
some other indirect method of handling specimens.
induced damage from cutting and grinding may not be re-
5.2.6 Methanol is a cumulative poison hazard. Where etha-
moved if preparation is stopped after a 600-grit SiC grind and
nolormethanolarelistedasalternates,ethanolisthepreferred
electropolishing times are short.
solvent. Methanol should be used in a properly designed
4.2.10 A large number of electrolytes may be needed to
chemical fume hood.
polish the variety of metals encountered by a given laboratory.
5.2.7 All spills should be cleaned up and disposed of
Considerable time may be required to develop a procedure for
properly, no matter how small the spill.
a new alloy.
5.2.8 Properly dispose of all solutions that are not identified
by composition and concentration.
5. General Safety Precautions
5.2.9 Store, handle, and dispose of chemicals according to
5.1 Beforeusingormixinganychemicals,allproductlabels
the manufacturer’s recommendations. Observe printed cau-
and pertinent Material Safety Data Sheets (MSDS) should be
tions on reagent containers.
read and understood concerning all of the hazards and safety
5.2.10 Information pertaining to the toxicity hazards and
precautions to be observed. Users should be aware of the type
working precautions of chemicals, solvents, acids, bases, and
of hazards involved in the use of all chemicals used, including
so forth, being used (such as MSDS) should be available for
those hazards that are immediate, long-term, visible, invisible,
rapid consultation.
and with or without odors.
5.1.1 Consult the product labels and MSDS for recommen- 5.3 Many of the electrolytes in the following listing can be
dations concerning proper protective clothing. exceedingly dangerous if carelessly handled. The pertinent
5.1.2 All chemicals are potentially dangerous. All persons safety precautions for each class of electrolyte should be read
using any electrolyte should be thoroughly familiar with all of before any electrolyte is mixed or used.
the chemicals involved and the proper procedure for handling,
5.4 Electrolytes containing perchloric acid and acetic anhy-
mixing, and disposing of each chemical, as well as any
dride are very dangerous to mix and may be unpredictable in
combinations of those chemicals.
use. Many industrial firms and research laboratories forbid the
5.1.3 When pouring, mixing, or etching, always use the
use of such mixtures. Certain cities also have ordinances
properprotectiveequipment(glasses,gloves,apron,etc.)andit
prohibiting the use of such potentially explosive mixtures.
is strongly recommended to always work under a certified and
These facts are considered sufficient reason for recommending
testedfumehood.Thisisimperativewithetchantsthatgiveoff
against their use.
noxious odors or toxic vapors. In particular, note that solutions
5.5 Mixtures of oxidizable organic compounds and power-
containing perchloric acid must be mixed and used in an
ful oxidizing agents are always potentially dangerous. After
exclusive hood equipped with a wash down feature to avoid
some use, any electrolyte will become heavily laden with ions
accumulation of explosive perchlorates.
of the metals polished. These ions may interfere with further
5.1.4 Table 1 includes specific safety precautions for the
polishing or catalyze the decomposition of the electrolyte.The
mixing or use of some electrolytes. The user should take care
electrolyte then must be discarded in accordance with appro-
to observe each of these specific precautions.
priate regulations.
5.2 Somebasicsuggestionsforthehandlinganddisposalof
electrolytes and their ingredients are as follows: 5.6 Most electrolytes (with few exceptions) should be
5.2.1 As previously stated, it is good practice to always mixed and stored in clean glass containers and never be in
work under a certified fume hood when mixing and utilizing contact with foreign materials or organic compounds. The
any electrolyte and it is imperative with those electrolytes that exceptions are those electrolytes containing fluorides and
give off noxious odors or toxic vapor. Additionally, the strong alkaline solutions that should be mixed and stored in
electrolytes in Groups I and II must be treated with extra polyethylene or other appropriate material containers. Electro-
caution because dried perchlorates can accumulate in hood lytes must never be allowed to become concentrated by
ductwork and on work surfaces creating the potential for a evaporation.All electrolytes should be discarded appropriately
powerful accidental explosion. Therefore, these electrolytes as soon as they have exceeded their immediate usefulness.
E1558 − 09 (2021)
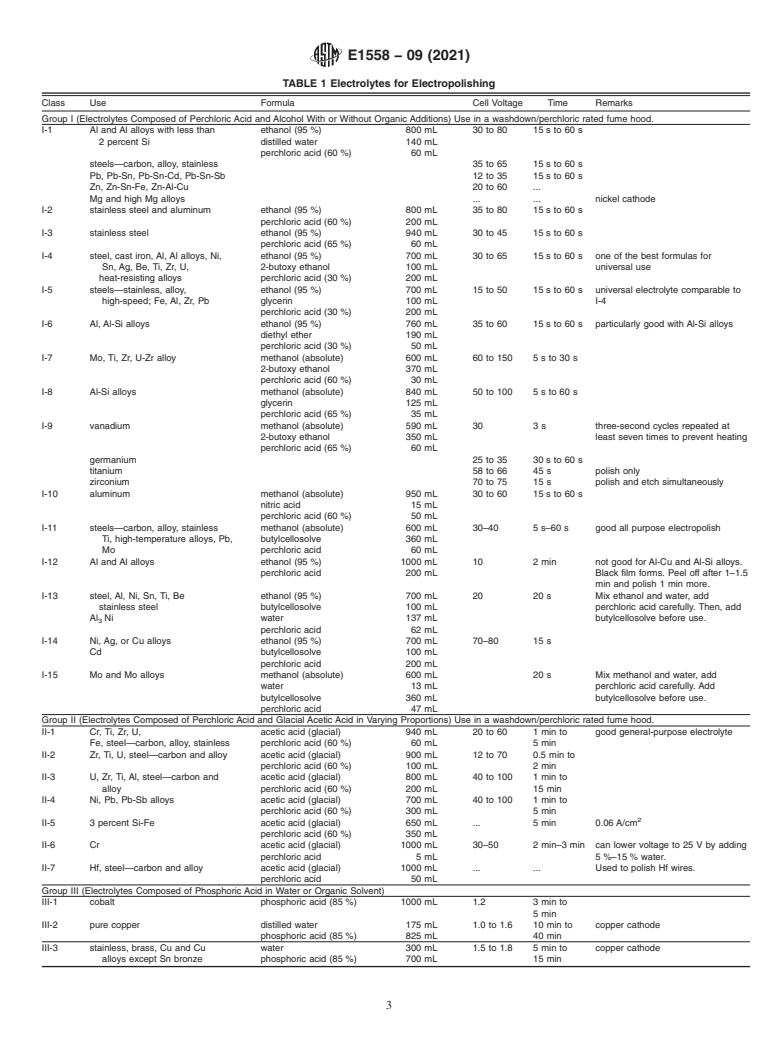
TABLE 1 Electrolytes for Electropolishing
Class Use Formula Cell Voltage Time Remarks
Group I (Electrolytes Composed of Perchloric Acid and Alcohol With or Without Organic Additions) Use in a washdown/perchloric rated fume hood.
I-1 Al and Al alloys with less than ethanol (95 %) 800 mL 30 to 80 15 s to 60 s
2 percent Si distilled water 140 mL
perchloric acid (60 %) 60 mL
steels—carbon, alloy, stainless 35 to 65 15 s to 60 s
Pb, Pb-Sn, Pb-Sn-Cd, Pb-Sn-Sb 12 to 35 15 s to 60 s
Zn, Zn-Sn-Fe, Zn-Al-Cu 20 to 60 .
Mg and high Mg alloys . . nickel cathode
I-2 stainless steel and aluminum ethanol (95 %) 800 mL 35 to 80 15 s to 60 s
perchloric acid (60 %) 200 mL
I-3 stainless steel ethanol (95 %) 940 mL 30 to 45 15 s to 60 s
perchloric acid (65 %) 60 mL
I-4 steel, cast iron, Al, Al alloys, Ni, ethanol (95 %) 700 mL 30 to 65 15 s to 60 s one of the best formulas for
Sn, Ag, Be, Ti, Zr, U, 2-butoxy ethanol 100 mL universal use
heat-resisting alloys perchloric acid (30 %) 200 mL
I-5 steels—stainless, alloy, ethanol (95 %) 700 mL 15 to 50 15 s to 60 s universal electrolyte comparable to
high-speed; Fe, Al, Zr, Pb glycerin 100 mL I-4
perchloric acid (30 %) 200 mL
I-6 Al, Al-Si alloys ethanol (95 %) 760 mL 35 to 60 15 s to 60 s particularly good with Al-Si alloys
diethyl ether 190 mL
perchloric acid (30 %) 50 mL
I-7 Mo, Ti, Zr, U-Zr alloy methanol (absolute) 600 mL 60 to 150 5 s to 30 s
2-butoxy ethanol 370 mL
perchloric acid (60 %) 30 mL
I-8 Al-Si alloys methanol (absolute) 840 mL 50 to 100 5 s to 60 s
glycerin 125 mL
perchloric acid (65 %) 35 mL
I-9 vanadium methanol (absolute) 590 mL 30 3 s three-second cycles repeated at
2-butoxy ethanol 350 mL least seven times to prevent heating
perchloric acid (65 %) 60 mL
germanium 25 to 35 30sto60s
titanium 58 to 66 45 s polish only
zirconium 70 to 75 15 s polish and etch simultaneously
I-10 aluminum methanol (absolute) 950 mL 30 to 60 15 s to 60 s
nitric acid 15 mL
perchloric acid (60 %) 50 mL
I-11 steels—carbon, alloy, stainless methanol (absolute) 600 mL 30–40 5 s–60 s good all purpose electropolish
Ti, high-temperature alloys, Pb, butylcellosolve 360 mL
Mo perchloric acid 60 mL
I-12 Al and Al alloys ethanol (95 %) 1000 mL 10 2 min not good for Al-Cu and Al-Si alloys.
perchloric acid 200 mL Black film forms. Peel off after 1–1.5
min and polish 1 min more.
I-13 steel, Al, Ni, Sn, Ti, Be ethanol (95 %) 700 mL 20 20 s Mix ethanol and water, add
stainless steel butylcellosolve 100 mL perchloric acid carefully. Then, add
Al Ni water 137 mL butylcellosolve before use.
perchloric acid 62 mL
I-14 Ni, Ag, or Cu alloys ethanol (95 %) 700 mL 70–80 15 s
Cd butylcellosolve 100 mL
perchloric acid 200 mL
I-15 Mo and Mo alloys methanol (absolute) 600 mL 20 s Mix methanol and water, add
water 13 mL perchloric acid carefully. Add
butylcellosolve 360 mL butylcellosolve before use.
perchloric acid 47 mL
Group II (Electrolytes Composed of Perchloric Acid and Glacial Acetic Acid in Varying Proportions) Use in a washdown/perchloric rated fume hood.
II-1 Cr, Ti, Zr, U, acetic acid (glacial) 940 mL 20 to 60 1 min to good general-purpose electrolyte
Fe, steel—carbon, alloy, stainless perchloric acid (60 %) 60 mL 5min
II-2 Zr, Ti, U, steel—carbon and alloy acetic acid (glacial) 900 mL 12 to 70 0.5 min to
perchloric acid (60 %) 100 mL 2min
II-3 U, Zr, Ti, Al, steel—carbon and acetic acid (glacial) 800 mL 40 to 100 1 min to
alloy perchloric acid (60 %) 200 mL 15 min
II-4 Ni, Pb, Pb-Sb alloys acetic acid (glacial) 700 mL 40 to 100 1 min to
perchloric acid (60 %) 300 mL 5min
II-5 3 percent Si-Fe acetic acid (glacial) 650 mL .
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.