ASTM D3695-95(2007)
(Test Method)Standard Test Method for Volatile Alcohols in Water by Direct Aqueous-Injection Gas Chromatography
Standard Test Method for Volatile Alcohols in Water by Direct Aqueous-Injection Gas Chromatography
SIGNIFICANCE AND USE
The major organic constituents in industrial waste water need to be identified for support of effective in-plant or pollution control programs. Currently, the most practical means for tentatively identifying and measuring a range of volatile organic compounds is gas-liquid chromatography.
SCOPE
1.1 This test method covers a wide range of alcohols with various structures and boiling points that can be separated and detected quantitatively in water and waste water at a minimum detection limit of approximately 1 mg/L by aqueous-injection gas-liquid chromatography. This test method can also be used to detect other volatile organic compounds qualitatively. Organic acids, amines, and high boiling, highly polar compounds are not readily detectable under this set of conditions. For analysis of organics with similar functionalities, refer to other test methods in Volumes 11.01 and 11.02 of the Annual Book of ASTM Standards.
1.2 This test method utilizes the procedures and precautions as described in Practice D 2908. Utilize the procedures and precautions as described therein.
1.3 This test method has been used successfully with reagent grade Type II and natural chlorinated tap waters. It is the user's responsibility to assure the validity of this test method for any untested matrices.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
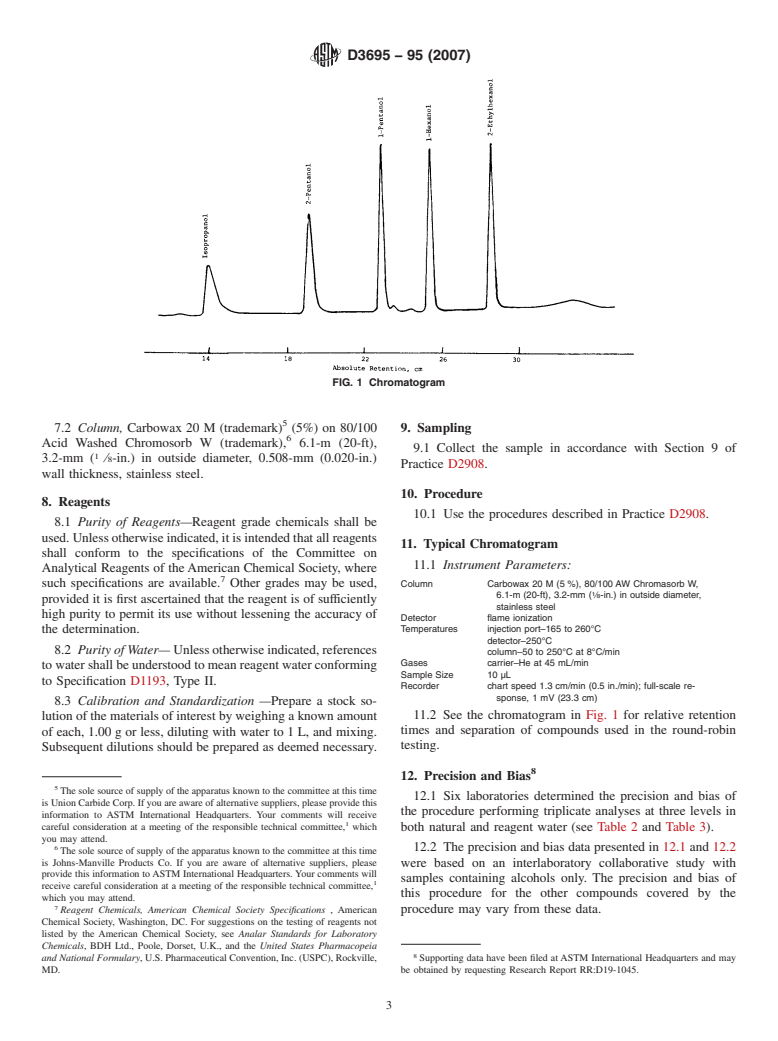
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3695 − 95(Reapproved 2007)
Standard Test Method for
Volatile Alcohols in Water by Direct Aqueous-Injection Gas
Chromatography
This standard is issued under the fixed designation D3695; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D2908 Practice for Measuring Volatile Organic Matter in
Water by Aqueous-Injection Gas Chromatography
1.1 This test method covers a wide range of alcohols with
D3856 Guide for Management Systems in Laboratories
various structures and boiling points that can be separated and
Engaged in Analysis of Water
detected quantitatively in water and waste water at a minimum
D4210 Practice for Intralaboratory Quality Control Proce-
detection limit of approximately 1 mg/L by aqueous-injection
2 dures and a Discussion on Reporting Low-Level Data
gas-liquid chromatography. This test method can also be used
(Withdrawn 2002)
to detect other volatile organic compounds qualitatively. Or-
E355 Practice for Gas Chromatography Terms and Relation-
ganic acids, amines, and high boiling, highly polar compounds
ships
are not readily detectable under this set of conditions. For
analysis of organics with similar functionalities, refer to other
3. Terminology
test methods in Volumes 11.01 and 11.02 of the Annual Book
3.1 Definitions—For definitions of terms used in this test
of ASTM Standards.
method, refer to Terminology D1129 and Practice E355.
1.2 This test method utilizes the procedures and precautions
as described in Practice D2908. Utilize the procedures and
4. Summary of Test Method
precautions as described therein.
4.1 An aliquot of an aqueous sample is directly injected into
1.3 This test method has been used successfully with
a gas chromatograph by means of a microlitre syringe. The
reagent grade Type II and natural chlorinated tap waters. It is
organic compounds in the sample are separated and eluted
the user’s responsibility to assure the validity of this test
from a chromatographic column into a flame ionization detec-
method for any untested matrices.
tor. The compounds are identified by relative retention time or
Kovats Index, and measured by direct comparison with corre-
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the sponding standard responses.
responsibility of the user of this standard to establish appro-
5. Significance and Use
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
5.1 The major organic constituents in industrial waste water
need to be identified for support of effective in-plant or
2. Referenced Documents
pollutioncontrolprograms.Currently,themostpracticalmeans
for tentatively identifying and measuring a range of volatile
2.1 ASTM Standards:
organic compounds is gas-liquid chromatography.
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water
6. Interferences
6.1 Sincethespecifiedcolumnandconditionsareapplicable
This test method is under the jurisdiction of ASTM Committee D19 on Water to numerous organics, the possibility of one or more compo-
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor
nents having identical retention times is always present.
Organic Substances in Water.
Therefore, the analyst must determine the qualitative identity
Current edition approved Dec. 1, 2007. Published January 2008. Originally
of the components of each peak by spectrometric techniques or
approved in 1978. Last previous edition approved in 2001 as D3695 – 95 (2001).
DOI: 10.1520/D3695-95R07.
a multi-column approach, or both, so that proper quantitation
Sugar, J.W., and Conway, R.A., “Gas-Liquid ChromatographicTechniques for
for those compounds of interest may be made. Refer to Table
Petrochemical Waste Water Analysis,’’ Journal of the Water Pollution Control
1 for relative retention data.
Federation, Vol 40, 1968, pp. 1622–1631.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3695 − 95 (2007)
TABLE 1 Continued
TABLE 1 Kovats Index and Relative Retention Data for Typical
A
Components
Kovats Relative
Component
B
Index (Ix) Retention
Kovats Relative
Component
B
Index (Ix) Retention
n-Butyl acrylate 1190 0.83
Methyl amyl alcohol 1190 0.83
Diethyl ether 580 0.17
n-Hexane 600 0.19 Diisobutyl ketone 1202 0.85
2-Ethylhexyl aldehyde 1210 0.87
Isopropyl ether 600 0.19
Ethylene oxide 700 0.20 Epichlorohydrin 1216 0.88
Acetaldehyde 700 0.20
2-Picoline 1222 0.91
n-Ethylmorpholine 1226 0.92
Vinyl ethyl ether 700 0.20
Styrene monomer 1240 0.95
n-Heptane 700 0.20
1,2-Trichlorethane 1244 0.96
Propylene oxide 737 0.22
Amyl alcohol 1260 1.00
Vinyl isobutyl ether 796 0.26
Acetone 796 0.26
Cyclohexanone 1260 1.00
n-Butyl chloride 796 0.26 1,3-Triethoxybutane 1260 1.00
Diethyl benzene 1275 1.04
Cyclohexene 808 0.27
2-Ethyl-1-butanol 1295 1.10
Acrolein 820 0.28
3-Picoline 1300 1.12
Methyl acetate 820 0.28
Vinyl n-butyl ether 833 0.29
4-Picoline 1303 1.14
Diisobutyl carbinol 1308 1.15
Octene-1 842 0.30
n-Butyraldehyde 865 0.32 1-Hexanol 1312 1.16
2-Ethylhexyl acetate 1322 1.20
Vinyl acetate 887 0.34
Isopropyl acetate 887 0.34
n-Hexyl ether 1325 1.21
Methyl ethyl ketone 908 0.36
Diacetone alcohol 1330 1.23
Ethylene chlorohydrin 1338 1.25
Ethyl acetate 912 0.37
2-Octanal 1341 1.26
Methanol 916 0.38
Isopropanol 935 0.39 1,3-Trichloropropane 1352 1.30
Dioxolane 943 0.40
Benzene 962 0.42 2-Methyl-5-ethyl pyridine 1354 1.31
Cyclohexanol 1354 1.31
Ethyl acrylate 978 0.44 Ethyl acetoacetate 1356 1.32
Iso-octanol (Isomers) 1362–1386 1.35–1.45
Isopropenyl acetate 983 0.45
Methyl n-propyl ketone 983 0.45
Dichloro isopropyl ether 1362 1.35
Methyl vinyl acetate 992 0.46
2-Ethyl-1-hexanol 1364 1.36
Ethanol 1000 0.47
2-Ethylhexyl acrylate 1376 1.40
Dichloroethyl ether 1384 1.44
Acrylonitrile 1007 0.48
Propyl acetate 1007 0.48 Tetralin 1388 1.45
2-Methylpentaldehyde 1026 0.51
Glycol diacetate 1392 1.46
n-Butyl ether 1026 0.51
n-Octanol 1402 1.51
Methyl isobutyl ketone 1035 0.52
Isophorone 1420 1.59
Isobutyl acetate 1035 0.52 Styrene oxide 1423 1.60
Ethylene glycol 1430 1.63
2-Ethylbutyraldehyde 1042 0.53
Acetonitrile 1050 0.54
Acetophenone 1435 1.65
1,2-Dichloropropane 1056 0.55
sec-Butyl alcohol 1056 0.55 Diethyl succinate 1441 1.67
Methyl acetoacetate 1443 1.69
Diethyl maleate 1460 1.79
Propylene dichloride 1065 0.57
n-Decyl alcohol 1483 1.85
2,3-Pentanedione 1080 0.60
Toluene 1080 0.60
n-Butyl acetate 1080 0.60 Methylbenzyl alcohol 1486 1.86
2-(2-Butoxy) ethoxyethyl ace- 1486 1.86
Ethylene dichloride 1092 0.62
tate
n-Propanol 1100 0.63
A
Gas Chromatographic Data Compilation, ASTM AMD 25A-51, ASTM, 1971.
Crotonaldehyde 1110 0.65 B
Relative to amyl alcohol.
Paraldehyde 1118 0.66
1,4-Dioxane 1118 0.66
Isobutanol 1137 0.70
Mesityl oxide 1137 0.70
n-Methylmorpholene 1142 0.72
Methyl amyl acetate 1150 0.73
7. Apparatus
2-Pentanol 1157 0.74
primary-Amyl acetate 1157–1185 0.74–0.82
7.1 Gas Chromatograph and Accessory Equipment, de-
(Isomers)
scribed in Practice D2908, Sections 7.1 through 7.6, is used for
p-Xylene 1160 0.75 this analysis.
Ethyl benzene 1160 0.75
Ethylidene acetone 1170 0.77
Methyl isoamyl ketone 1173 0.78
n-Butanol 1185 0.82
D3695 − 95 (2007)
FIG. 1 Chromatogram
7.2 Col
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.