ASTM D3223-95
(Test Method)Standard Test Method for Total Mercury in Water
Standard Test Method for Total Mercury in Water
SCOPE
1.1 This test method covers the determination of total mercury in water in the range from 0.5 to 10.0 g Hg/L (1). The test method is applicable to fresh waters, saline waters, and some industrial and sewage effluents. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.1.1 The analyst should recognize that the precision and bias of this standard may be affected by the other constituents in all waters, as tap, industrial, river, and wastewaters. The cold vapor atomic absorption measurement portion of this method is applicable to the analysis of materials other than water (sediments, biological materials, tissues, etc.) if, and only if, an initial procedure for digesting and oxidizing the sample is carried out, ensuring that the mercury in the sample is converted to the mercuric ion, and is dissolved in aqueous media (2,3).
1.2 Both organic and inorganic mercury compounds may be analyzed by this procedure if they are first converted to mercuric ions. Using potassium persulfate and potassium permanganate as oxidants, and a digestion temperature of 95°C, approximately 100 % recovery of organomercury compounds can be obtained (2,4).
1.3 The range of the test method may be changed by instrument or recorder expansion or both, and by using a larger volume of sample.
1.4 A method for the disposal of mercury-containing wastes is also presented (Appendix X1) (5).
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see 7.8 and 10.8.2.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or
withdrawn. Contact ASTM International (www.astm.org) for the latest information.
Designation: D 3223 – 95
AMERICAN SOCIETY FOR TESTING AND MATERIALS
100 Barr Harbor Dr., West Conshohocken, PA 19428
Reprinted from the Annual Book of ASTM Standards. Copyright ASTM
Standard Test Method for
1
Total Mercury in Water
This standard is issued under the fixed designation D 3223; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense. Consult the DoD Index of Specifications and
Standards for the specific year of issue which has been adopted by the Department of Defense.
1. Scope statements, see Note 3 and Note 8.
2
1.1 This test method covers the determination of total
3 2. Referenced Documents
mercury in water in the range from 0.5 to 10.0 μg Hg/L (1).
The test method is applicable to fresh waters, saline waters, and 2.1 ASTM Standards:
4
some industrial and sewage effluents. It is the user’s responsi- D 512 Test Methods for Chloride Ion in Water
4
bility to ensure the validity of this test method for waters of D 1129 Terminology Relating to Water
4
untested matrices. D 1193 Specification for Reagent Water
D 1245 Practice for Examination of Water-Formed Deposits
1.1.1 The analyst should recognize that the precision and
5
by Chemical Microscopy
bias of this standard may be affected by the other constituents
D 1252 Test Methods for Chemical Oxygen Demand
in all waters, as tap, industrial, river, and wastewaters. The cold
5
(Dichromate Oxygen Demand) of Water
vapor atomic absorption measurement portion of this method is
4
D 1426 Test Methods for Ammonia Nitrogen in Water
applicable to the analysis of materials other than water (sedi-
D 3370 Practices for Sampling Water from Closed Con-
ments, biological materials, tissues, etc.) if, and only if, an
4
duits
initial procedure for digesting and oxidizing the sample is
D 4691 Practice for Measuring Elements in Water by Flame
carried out, ensuring that the mercury in the sample is
4
Atomic Absorption Spectrophotometry
converted to the mercuric ion, and is dissolved in aqueous
D 4841 Practice for Estimation of Holding Time for Water
media (2,3).
4
Samples Containing Organic and Inorganic Constituents
1.2 Both organic and inorganic mercury compounds may be
analyzed by this procedure if they are first converted to
3. Terminology
mercuric ions. Using potassium persulfate and potassium
permanganate as oxidants, and a digestion temperature of
3.1 Definitions—For definitions of terms used in this test
95°C, approximately 100 % recovery of organomercury com-
method, refer to Terminology D 1129.
pounds can be obtained (2,4).
1.3 The range of the test method may be changed by
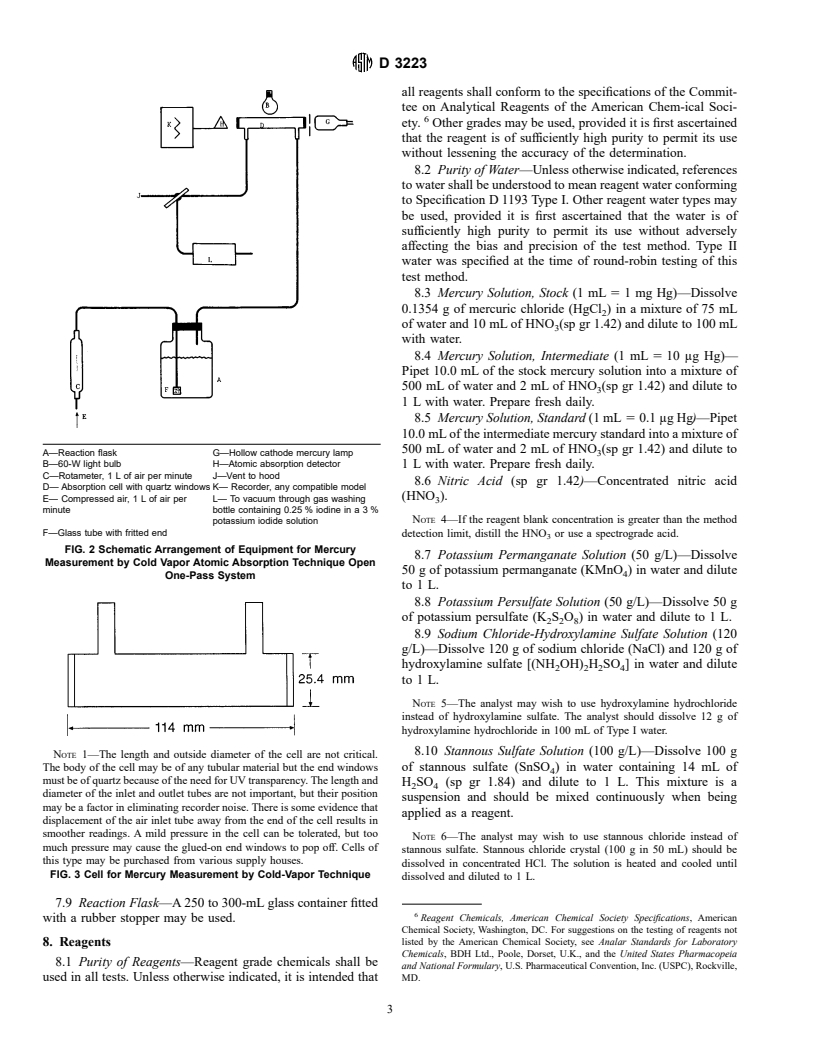
4. Summary of Test Method
instrument or recorder expansion or both, and by using a larger
4.1 The test method consists of a wet chemical oxidation
volume of sample.
which converts all mercury to the mercuric ion; reduction of
1.4 A method for the disposal of mercury-containing wastes
mercuric ions to metallic mercury, followed by a cold vapor
is also presented (Appendix X1) (5).
atomic absorption analysis (1,2). A general guide for flame and
1.5 This standard does not purport to address all of the
vapor generation atomic absorption applications is given in
safety concerns, if any, associated with its use. It is the
Practice D 4691.
responsibility of the user of this standard to establish appro-
4.2 Cold vapor atomic absorption analysis is a physical
priate safety and health practices and determine the applica-
method based on the absorption of ultraviolet radiation at a
bility of regulatory limitations prior to use. For specific hazard
wavelength of 253.7 nm by mercury vapor. The mercury is
reduced to the elemental state and aerated from solution in
either a closed recirculating system or an open one-pass
1
This test method is under the jurisdiction of ASTM Committee D-19 on Water
system. The mercury vapor passes through a cell positioned in
and is the direct responsibility of Subcommittee D19.05 on Inorganic Constituents
in Water. the light path of an atomic absorption spectrophotometer.
Current edition approved Dec. 10, 1995. Published February 1996. Originally
Absorbance is measured as a function of mercury concentra-
published as D 3223–79. Last previous edition D 3223 – 91.
tion.
2
Adapted from research investigations by the U. S. Environmental Protection
Agency’s Analytical Quality Control Laboratory, Cincinnati, OH, and Region IV
Surveillance and Analysis Division, Chemical Services Branch, Athens, GA.
3 4
The boldface numbers in parentheses refer to the references at the end of this Annual Book of ASTM Stand
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.