ASTM C698-98
(Test Method)Standard Test Methods for Chemical, Mass Spectrometric, and Spectrochemical Analysis of Nuclear-Grade Mixed Oxides ((U, Pu)O2)
Standard Test Methods for Chemical, Mass Spectrometric, and Spectrochemical Analysis of Nuclear-Grade Mixed Oxides ((U, Pu)O<sub>2</sub>)
SCOPE
1.1 These test methods cover procedures for the chemical, mass spectrometric, and spectrochemical analysis of nuclear-grade mixed oxides, (U, Pu)O2, powders and pellets to determine compliance with specifications.
1.2 The analytical procedures appear in the following order: Sections Uranium by Controlled-Potential Coulometry 2 Plutonium by Controlled-Potential Coulometry 2 Plutonium by Amperometric Titration with Iron (II) 2 Nitrogen by Distillation Spectrophotometry Using Nessler Reagent 34 to 41 Carbon (Total) by Direct Combustion-Thermal Conductivity 42 to 53 Total Chlorine and Fluorine by Pyrohydrolysis 54 to 61 Sulfur by Distillation-Spectrophotometry 62 to 70 Moisture by the Coulometric, Electrolytic Moisture Analyzer 71 to 78 Isotopic Composition by Mass Spectrometry 3 Rare Earths by Copper Spark Spectroscopy 87 to 94 Trace Impurities by Carrier Distillation Spectroscopy 95 to 104 Impurities by Spark-Source Mass Spectrography 105 to 111 Total Gas in Reactor-Grade Mixed Dioxide Pellets 112 to 119 Tungsten by Dithio Spectrophotometry 120 to 128 Rare Earth Elements by Spectroscopy 129 to 132 Plutonium-238 Isotopic Abundance by Alpha Spectrometry 133 to 140 Uranium and Plutonium Isotopic Analysis by Mass Spectrometry 141 to 149 Oxygen-to-Metal Atom Ratio by Gravimetry 150 to 158
1.3 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. (For specific safeguard and safety precaution statements, see Sections 38, 47, 99, and 155 and 145.6.1.)
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: C 698 – 98
Standard Test Methods for
Chemical, Mass Spectrometric, and Spectrochemical
Analysis of Nuclear-Grade Mixed Oxides ((U, Pu)O )
This standard is issued under the fixed designation C 698; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope Dioxide Powders and Pellets
C 833 Specification for Sintered (Uranium-Plutonium) Di-
1.1 These test methods cover procedures for the chemical,
oxide Pellets
mass spectrometric, and spectrochemical analysis of nuclear-
C 852 Guide for Design Criteria for Plutonium Gloveboxes
grade mixed oxides, (U, Pu)O , powders and pellets to deter-
C 1009 Guide for Establishing a Quality Assurance Pro-
mine compliance with specifications.
gram for Analytical Chemistry Laboratories Within the
1.2 The analytical procedures appear in the following order:
Nuclear Industry
Sections
C 1068 Guide for Qualification of Measurement Methods
Uranium by Controlled-Potential Coulometry
Plutonium by Controlled-Potential Coulometry
by a Laboratory Within the Nuclear Industry
Plutonium by Amperometric Titration with Iron (II)
C 1108 Test Method for Plutonium by Controlled-Potential
Nitrogen by Distillation Spectrophotometry Using Nessler Re- 7to 14
Coulometry
agent
Carbon (Total) by Direct Combustion-Thermal Conductivity 15 to 26
C 1128 Guide for Preparation of Working Reference Mate-
Total Chlorine and Fluorine by Pyrohydrolysis 27 to 34
rials for Use in the Analysis of Nuclear Fuel Cycle
Sulfur by Distillation-Spectrophotometry 35 to 43
Materials
Moisture by the Coulometric, Electrolytic Moisture Analyzer 44 to 51
Isotopic Composition by Mass Spectrometry
C 1156 Guide for Establishing Calibration for a Measure-
Rare Earths by Copper Spark Spectroscopy 52 to 59
ment Method Used to Analyze Nuclear Fuel Cycle Mate-
Trace Impurities by Carrier Distillation Spectroscopy 60 to 69
Impurities by Spark-Source Mass Spectrography 70 to 76 rials
Total Gas in Reactor-Grade Mixed Dioxide Pellets 77 to 84
C 1165 Test Method for Determining Plutonium by
Tungsten by Dithiol-Spectrophotometry 85 to 93
Controlled-Potential Coulometry in H SO at a Platinum
2 4
Rare Earth Elements by Spectroscopy 94 to 97
Plutonium-238 Isotopic Abundance by Alpha Spectrometry 98 to 105 Working Electrode
Americium-241 in Plutonium by Gamma-Ray Spectrometry
C 1168 Practice for Preparation and Dissolution of Pluto-
Uranium and Plutonium Isotopic Analysis by Mass Spectrom- 106 to 114
nium Materials for Analysis
etry
Oxygen-to-Metal Atom Ratio by Gravimetry 115 to 123 C 1204 Test Method for Uranium in the Presence of Pluto-
nium by Iron (II) Reduction in Phosphoric Acid Followed
1.3 This standard does not purport to address all of the
by Chromium (VI) Titration
safety concerns, if any, associated with its use. It is the
C 1206 Test Method for Plutonium by Iron (II)/Chromium
responsibility of the user of this standard to establish appro-
(VI) Amperometric Titration
priate safety and health practices and determine the applica-
C 1210 Guide for Establishing a Measurement System
bility of regulatory limitations prior to use. (For specific
Quality Control Program for Analytical Chemistry Labo-
safeguard and safety precaution statements, see Sections 11,
ratories Within the Nuclear Industry
20, 64, and 120 and 110.6.1.)
C 1268 Test Method for Quantitative Determination of
Americium 241 in Plutonium by Gamma-Ray Spectrom-
2. Referenced Documents
etry
2.1 ASTM Standards:
C 1297 Guide for Qualification of Laboratory Analysts for
C 697 Test Methods for Chemical, Mass Spectrometric, and
the Analysis of Nuclear Fuel Cycle Materials
Spectrochemical Analysis of Nuclear-Grade Plutonium
D 1193 Specification for Reagent Water
E 60 Practice for Photometric and Spectrophotometric
Methods for Chemical Analysis of Metals
These test methods are under the jurisdiction of ASTM Committee C-26 on
E 115 Practices for Photographic Processing in Optical
Nuclear Fuel Cycle and are the direct responsibility of Subcommittee C26.05 on
Methods of Test.
Current edition approved Feb. 10, 1998. Published June 1998. Originally pub-
lished as C698 – 72. Last previous edition C698 – 92. Annual Book of ASTM Standards, Vol 12.01.
2 5
Discontinued as of Nov. 15, 1992. Annual Book of ASTM Standards, Vol 11.01.
3 6
Discontinued as of May 30, 1980. Annual Book of ASTM Standards, Vol 03.05.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
C 698
Emission Spectrographic Analysis 5.2.1 The materials [nuclear grade mixed oxides (U, Pu)O
E 116 Practice for Photographic Photometry in Spectro- powders and pellets] to which these test methods apply are
chemical Analysis subject to nuclear safeguards regulations governing their pos-
session and use. The following analytical procedures in these
3. Significance and Use
test methods have been designated as technically acceptable for
3.1 Mixed oxide, a mixture of uranium and plutonium
generating safeguards accountability measurement data: Ura-
oxides, is used as a nuclear-reactor fuel in the form of pellets. nium by Controlled Potential Coulometry; Plutonium by
The plutonium content may be up to 10 weight %, and the
Controlled-Potential Coulometry; Plutonium by Amperometric
diluent uranium may be of any U enrichment. In order to be Titration with Iron(II); Plutonium-238 Isotopic Abundance by
suitable for use as a nuclear fuel, the material must meet certain
Alpha Spectrometry; and Uranium and Plutonium Isotopic
criteria for combined uranium and plutonium content, effective Analysis by Mass Spectrometry.
fissile content, and impurity content as described in Specifica-
5.2.2 When used in conjunction with appropriate certified
tion C 833. reference materials (CRMs), these procedures can demonstrate
3.1.1 The material is assayed for uranium and plutonium to
traceability to the national measurements base. However,
determine whether the plutonium content is as specified by the
adherence to these procedures does not automatically guaran-
purchaser, and whether the material contains the minimum tee regulatory acceptance of the resulting safeguards measure-
combined uranium and plutonium contents specified on a dry
ments. It remains the sole responsibility of the user of these test
weight basis. methods to assure that its application to safeguards has the
3.1.2 Determination of the isotopic content of the plutonium
approval of the proper regulatory authorities.
and uranium in the mixed oxide is made to establish whether
6. Sampling and Dissolution
the effective fissile content is in compliance with the purchas-
er’s specifications. 6.1 Criteria for sampling this material are given in Specifi-
3.1.3 Impurity content is determined to ensure that the
cation C 833.
maximum concentration limit of certain impurity elements is 6.2 Samples can be dissolved using the appropriate disso-
not exceeded. Determination of impurities is also required for
lution techniques described in Practice C 1168.
calculation of the equivalent boron content (EBC).
URANIUM IN THE PRESENCE OF PLUTONIUM BY
4. Reagents
POTENTIOMETRIC TITRATION
4.1 Purity of Reagents—Reagent grade chemicals shall be
(This test method was discontinued in 1992 and replaced by
used in all tests. Unless otherwise indicated, it is intended that
Test Method C 1204.)
all reagents shall conform to the specifications of the Commit-
PLUTONIUM BY CONTROLLED POTENTIAL
tee on Analytical Reagents of the American Chemical Society,
COULOMETRY
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
(This test method was discontinued in 1992 and replaced by
sufficiently high purity to permit its use without lessening the
Test Method C 1165.)
accuracy of the determination.
PLUTONIUM BY CONTROLLED-POTENTIAL
4.2 Purity of Water— Unless otherwise indicated, refer-
COULOMETRY
ences to water shall be understood to mean reagent water
conforming to Specification D 1193.
(With appropriate sample preparation, controlled-potential
coulometric measurement as described in Test Method C 1108
5. Safety Precautions
may be used for plutonium determination.)
5.1 Since plutonium- and uranium-bearing materials are
PLUTONIUM BY AMPEROMETRIC TITRATION
radioactive and toxic, adequate laboratory facilities, gloved
WITH IRON(II)
boxes, fume hoods, etc., along with safe techniques must be
used in handling samples containing these materials. A detailed
(This test method was discontinued in 1992 and replaced by
discussion of all the precautions necessary is beyond the scope
Test Method C 1206.)
of these test methods; however, personnel who handle these
NITROGEN BY DISTILLATION
materials should be familiar with such safe handling practices
SPECTROPHOTOMETRY USING NESSLER
as are given in Guide C 852 and in Refs (1) through (3).
REAGENT
5.2 Committee C-26 Safeguards Statement:
7. Scope
“Reagent Chemicals, American Chemical Society Specifications,” Am. Chemi-
7.1 This test method covers the determination of 5 to 100
cal Soc., Washington, DC. For suggestions on the testing of reagents not listed by
μg/g of nitride nitrogen in mixtures of plutonium and uranium
the American Chemical Society, see “Reagent Chemicals and Standards,” by Joseph
oxides in either pellet or powder form.
Rosin, D. Van Nostrand Co., Inc., New York, NY, and the “United States
Pharmacopeia.”
The boldface numbers in parentheses refer to the list of references at the end of
8. Summary of Test Method
these test methods.
8.1 The sample is dissolved in hydrochloric acid by the
Based upon Committee C-26 Safeguards Matrix (C 1009, C 1068, C 1128,
C 1156, C 1210, C 1297). sealed tube test method or by phosphoric acid-hydrofluoric
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
C 698
acid solution, after which the solution is made basic with prior to use, and ( 2) avoid contamination of the atmosphere in
sodium hydroxide and nitrogen is separated as ammonia by the vicinity of the test by ammonia or other volatile nitrog-
steam distillation. Nessler reagent is added to the distillate to enous compounds.
form the yellow ammonium complex and the absorbance of the
12. Procedure
solution is measured at approximately 430 nm (4, 5).
12.1 Dissolution of Sample:
9. Apparatus 12.1.1 Transfer a weighed sample, in the range from 1.0 to
1.5 g, to a 50-mL beaker.
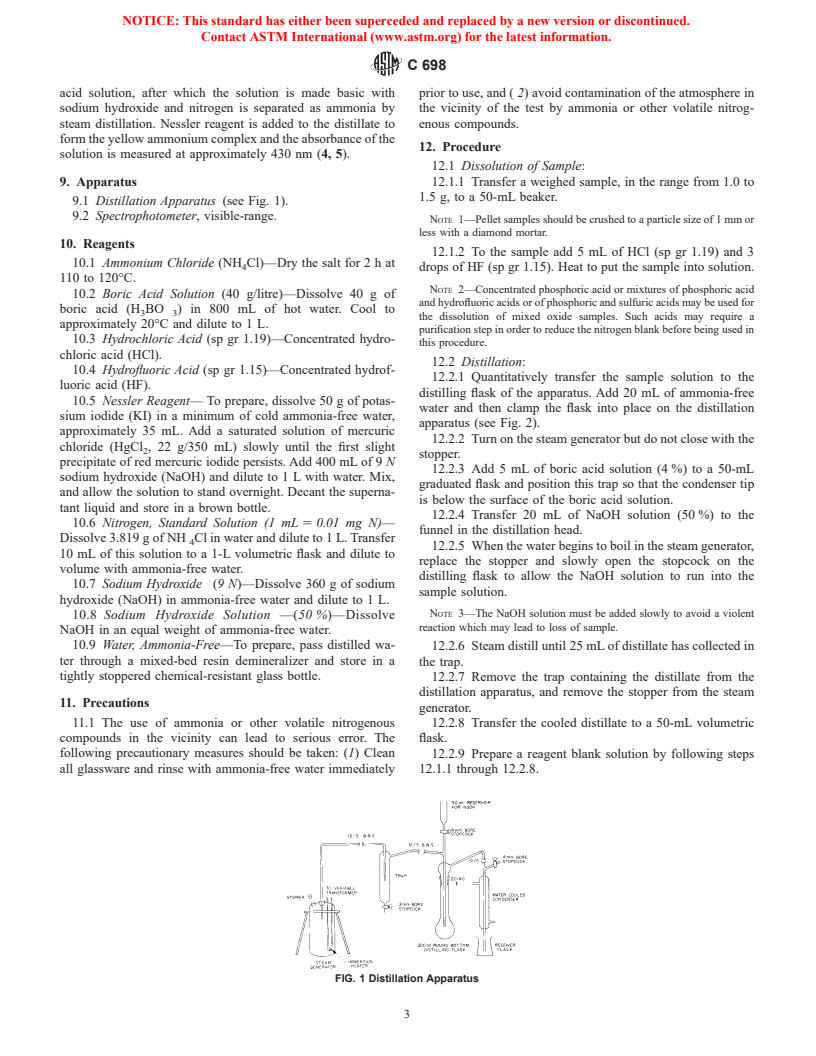
9.1 Distillation Apparatus (see Fig. 1).
9.2 Spectrophotometer, visible-range.
NOTE 1—Pellet samples should be crushed to a particle size of 1 mm or
less with a diamond mortar.
10. Reagents
12.1.2 To the sample add 5 mL of HCl (sp gr 1.19) and 3
10.1 Ammonium Chloride (NH Cl)—Dry the salt for2hat
drops of HF (sp gr 1.15). Heat to put the sample into solution.
110 to 120°C.
NOTE 2—Concentrated phosphoric acid or mixtures of phosphoric acid
10.2 Boric Acid Solution (40 g/litre)—Dissolve 40 g of
and hydrofluoric acids or of phosphoric and sulfuric acids may be used for
boric acid (H BO ) in 800 mL of hot water. Cool to
3 3
the dissolution of mixed oxide samples. Such acids may require a
approximately 20°C and dilute to 1 L.
purification step in order to reduce the nitrogen blank before being used in
10.3 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
this procedure.
chloric acid (HCl).
12.2 Distillation:
10.4 Hydrofluoric Acid (sp gr 1.15)—Concentrated hydrof-
12.2.1 Quantitatively transfer the sample solution to the
luoric acid (HF).
distilling flask of the apparatus. Add 20 mL of ammonia-free
10.5 Nessler Reagent— To prepare, dissolve 50 g of potas-
water and then clamp the flask into place on the distillation
sium iodide (KI) in a minimum of cold ammonia-free water,
apparatus (see Fig. 2).
approximately 35 mL. Add a saturated solution of mercuric
12.2.2 Turn on the steam generator but do not close with the
chloride (HgCl , 22 g/350 mL) slowly until the first slight
stopper.
precipitate of red mercuric iodide persists. Add 400 mL of 9 N
12.2.3 Add 5 mL of boric acid solution (4 %) to a 50-mL
sodium hydroxide (NaOH) and dilute to 1 L with water. Mix,
graduated flask and position this trap so that the condenser tip
and allow the solution to stand overnight. Decant the superna-
is below the surface of the boric acid solution.
tant liquid and store in a brown bottle.
12.2.4 Transfer 20 mL of NaOH solution (50 %) to the
10.6 Nitrogen, Standard Solution (1 mL 5 0.01 mg N)—
funnel in the distillation head.
Dissolve 3.819 g of NH Cl in water and dilute to 1 L. Transfer
12.2.5 When the water begins to boil in the steam generator,
10 mL of this solution to a 1-L volumetric flask and dilute to
replace the stopper and slowly open the stopcock on the
volume with ammonia-free water.
distilling flask to allow the NaOH solution to run into the
10.7 Sodium Hydroxide (9N)—Dissolve 360 g of sodium
sample solution.
hydroxide (NaOH) in ammonia-free water and dilute to 1 L.
NOTE 3—The NaOH solution must be added slowly to avoid a violent
10.8 Sodium Hydroxide Solution —(50 %)—Dissolve
reaction which may lead to loss of sample.
NaOH in an equal weight of ammonia-free water.
10.9 Water, Ammonia-Free—To prepare, pass distilled wa- 12.2.6 Steam distill until 25 mL of distillate has collected in
ter through a mixed-bed resin demineralizer and store in a
the trap.
tightly stoppered chemical-resistant glass bottle. 12.2.7 Remove the trap containing the distillate from the
distillation apparatus, and remove the stopper from the steam
11. Precautions
generator.
11.1 The use of ammonia or other volatile nitrogenous 12.2.8 Transfer the cooled distillate to a 50-mL volumetric
compounds in the vicinity can lead to serious error. The flask.
following precautionary measures should be taken: (1) Clean 12.2.9 Prepare a reagent blank solution by following steps
all glassware and rinse with ammonia-free water immediately 12.1.1 through 12.2.8.
FIG. 1 Distillation Apparatus
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
C 698
FIG. 2 Quartz Reaction Tube
12.3 Measurement of Nitrogen: an induction heating furnace. Trace
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.