ASTM D1434-82(2009)e1
(Test Method)Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting
Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting
SIGNIFICANCE AND USE
These measurements give semiquantitative estimates for the gas transmission of single pure gases through film and sheeting. Correlation of measured values with any given use, such as packaged contents protection, must be determined by experience. The gas transmission rate is affected by conditions not specifically provided for in these tests, such as moisture content (Note 2), plasticizer content, and nonhomogeneities. These tests do not include any provision for testing seals that may be involved in packaging applications.
Note 2—The tests are run using gas with 0 % moisture changes.
Interlaboratory testing has revealed that permeances measured by these procedures exhibit a strong dependence on the procedure being used, as well as on the laboratory performing the testing. Agreement with other methods is sometimes poor and may be material-dependent. The materials being tested often affect the between-laboratory precision. The causes of these variations are not known at this time. It is suggested that this method not be used for referee purposes unless purchaser and seller can both establish that they are measuring the same quantity to a mutually agreed upon level of precision.
Use of the permeability coefficient (involving conversion of the gas transmission rate to a unit thickness basis) is not recommended unless the thickness-to-transmission rate relationship is known from previous studies. Even in essentially homogeneous structures, variations in morphology (as indicated, for example, by density) and thermal history may influence permeability.
SCOPE
1.1 This test method covers the estimation of the steady-state rate of transmission of a gas through plastics in the form of film, sheeting, laminates, and plastic-coated papers or fabrics. This test method provides for the determination of (1) gas transmission rate (GTR), (2) permeance, and, in the case of homogeneous materials, (3) permeability.
1.2 Two procedures are provided:
1.2.1 Procedure M—Manometric.
1.2.2 Procedure V—Volumetric.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: D1434 − 82(Reapproved 2009)
Standard Test Method for
Determining Gas Permeability Characteristics of Plastic Film
and Sheeting
This standard is issued under the fixed designation D1434; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Units information was revised editorially in May 2009.
1. Scope film in unit time under the conditions of test. The SI unit of
GTR is 1 mol/(m ·s). The test conditions, including tempera-
1.1 This test method covers the estimation of the steady-
ture and partial pressure of the gas on both sides of the film,
state rate of transmission of a gas through plastics in the form
must be stated. Other factors, such as relative humidity and
of film, sheeting, laminates, and plastic-coated papers or
hydrostatic pressure, that influence the transport of the gas
fabrics. This test method provides for the determination of (1)
must also be stated.The inch-pound unit of GTR, a commonly
gastransmissionrate(GTR), (2)permeance,and,inthecaseof
used unit of GTR, is 1 mL (STP)/(m ·d) at a pressure
homogeneous materials, (3) permeability.
differential of one atmosphere.
1.2 Two procedures are provided:
3.1.2 permeance, P—theratioofthegastransmissionrateto
1.2.1 Procedure M—Manometric.
the difference in partial pressure of the gas on the two sides of
1.2.2 Procedure V—Volumetric.
the film.The SI unit of permeance is 1 mol/ (m ·s·Pa).The test
1.3 The values stated in SI units are to be regarded as
conditions (see 5.1) must be stated.
standard. No other units of measurement are included in this
3.1.3 permeability, P—theproductofthepermeanceandthe
standard.
thickness of a film. The permeability is meaningful only for
1.4 This standard does not purport to address all of the
homogeneous materials, in which it is a property characteristic
safety concerns, if any, associated with its use. It is the
of the bulk material. This quantity should not be used unless
responsibility of the user of this standard to establish appro-
the constancy of the permeability has been verified using
priate safety and health practices and determine the applica-
severaldifferentthicknessesofthematerial.TheSIunitof Pis
bility of regulatory limitations prior to use.
1 mol/(m·s·Pa). The test conditions (see 3.1) must be stated.
NOTE 1—One millilitre (STP) is 44.62 µmol, one atmosphere is 0.1013
2. Referenced Documents
MPa, and one day is 86.4×10 s. GTR in SI units is obtained by
−10
2.1 ASTM Standards:
multiplying the value in inch-pound units by 5.160×10 . Additional
D618Practice for Conditioning Plastics for Testing units and conversions are shown in Appendix X1.
D1898Practice for Sampling of Plastics (Withdrawn 1998)
3.1.4 steady state—the state attained when the amount of
gas absorbed in the film is in equilibrium with the flux of gas
3. Terminology
throughthefilm.ForMethodV,thisisobtainedwhentheGTR
is constant.
3.1 Definitions of Terms Specific to This Standard:
3.1.1 gas transmission rate, GTR—the quantity of a given
gas passing through a unit of the parallel surfaces of a plastic 4. Summary of Test Method
4.1 The sample is mounted in a gas transmission cell so as
to form a sealed semibarrier between two chambers. One
ThistestmethodisunderthejurisdictionofASTMCommitteeF02onFlexible
chamber contains the test gas at a specific high pressure, and
Barrier Packaging and is the direct responsibility of Subcommittee F02.10 on
Permeation. theotherchamber,atalowerpressure,receivesthepermeating
Current edition approved May 1, 2009. Published June 2009. Originally
gas. Either of the following procedures is used:
approved in 1956. Last previous edition approved in 2003 as D1434–82 (2003).
4.1.1 Procedure M—In Procedure M the lower pressure
DOI: 10.1520/D1434-82R09E01.
chamber is initially evacuated and the transmission of the gas
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
through the test specimen is indicated by an increase in
Standards volume information, refer to the standard’s Document Summary page on
pressure.
the ASTM website.
4.1.2 Procedure V—In Procedure V the lower pressure
The last approved version of this historical standard is referenced on
www.astm.org. chamber is maintained near atmospheric pressure and the
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
D1434 − 82 (2009)
transmission of the gas through the test specimen is indicated
by a change in volume.
5. Significance and Use
5.1 Thesemeasurementsgivesemiquantitativeestimatesfor
the gas transmission of single pure gases through film and
sheeting. Correlation of measured values with any given use,
such as packaged contents protection, must be determined by
experience. The gas transmission rate is affected by conditions
not specifically provided for in these tests, such as moisture
content (Note 2), plasticizer content, and nonhomogeneities.
These tests do not include any provision for testing seals that
may be involved in packaging applications.
NOTE 2—The tests are run using gas with 0% moisture changes.
5.2 Interlaboratory testing has revealed that permeances
measured by these procedures exhibit a strong dependence on
FIG. 1 Manometric Gas Transmission Cell
the procedure being used, as well as on the laboratory
performing the testing. Agreement with other methods is
sometimes poor and may be material-dependent.The materials
being tested often affect the between-laboratory precision. The
8. Sampling
causes of these variations are not known at this time. It is
suggested that this method not be used for referee purposes
8.1 The techniques used in sampling a batch of material to
unless purchaser and seller can both establish that they are
be tested by these procedures must depend upon the kind of
measuringthesamequantitytoamutuallyagreeduponlevelof
information that is sought. Care should be taken to ensure that
precision.
samples represent conditions across the width and along the
5.3 Use of the permeability coefficient (involving conver- length of rolls of film. Practice D1898 provides guidelines for
sionofthegastransmissionratetoaunitthicknessbasis)isnot deciding what procedures to use in sampling a batch of
recommended unless the thickness-to-transmission rate rela- material. Enough specimens must be tested to ensure that the
information obtained is representative of the batch or other lot
tionship is known from previous studies. Even in essentially
homogeneous structures, variations in morphology (as size being tested.
indicated, for example, by density) and thermal history may
PROCEDURE M
influence permeability.
(Pressurechangesinthemanometriccellmaybedetermined
by either visual or automatic recording.)
6. Test Specimen
6.1 Thetestspecimenshallberepresentativeofthematerial, MANOMETRIC VISUAL DETERMINATION
free of wrinkles, creases, pinholes, and other imperfections,
and shall be of uniform thickness. The test specimen shall be
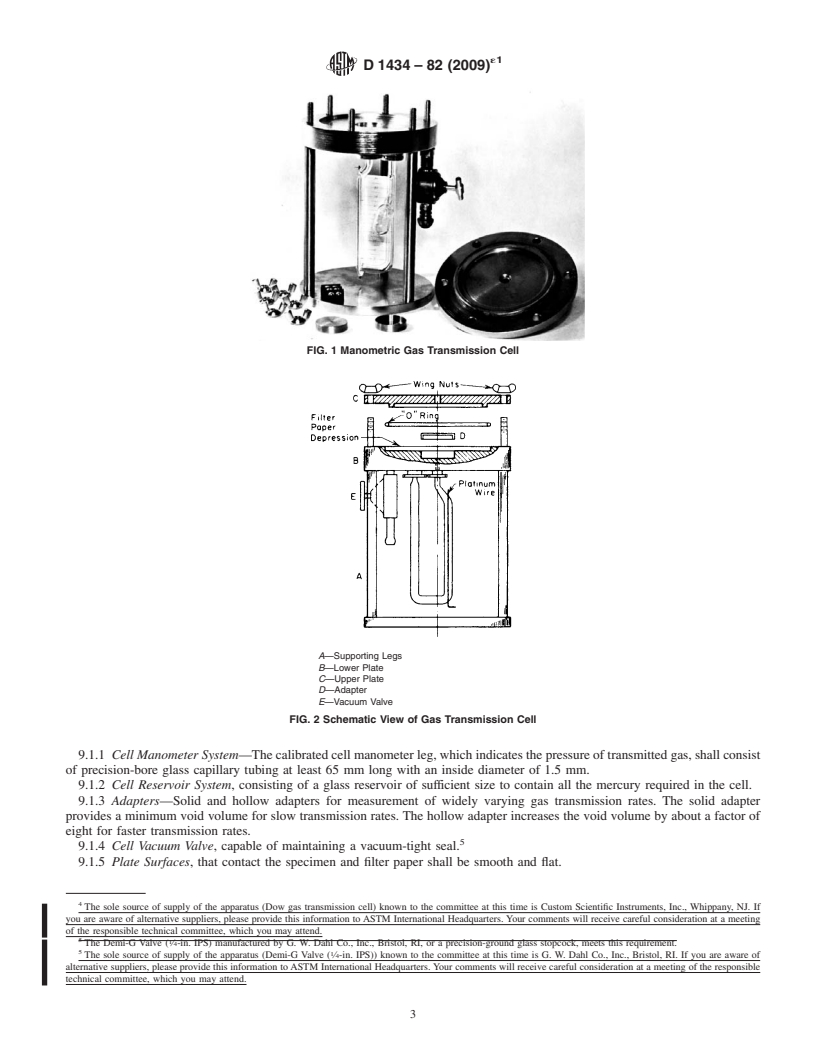
9. Apparatus
cuttoanappropriatesize(generallycircular)tofitthetestcell.
9.1 The apparatus shown in Fig. 1 and Fig. 2 consists of the
6.2 The thickness of the specimen shall be measured to the
following items:
nearest 2.5 µm with a calibrated dial gage (or equivalent) at a
9.1.1 Cell Manometer System—The calibrated cell manom-
minimum of five points distributed over the entire test area.
eter leg, which indicates the pressure of transmitted gas, shall
Maximum, minimum, and average values should be recorded.
consist of precision-bore glass capillary tubing at least 65 mm
An alternative measure of thickness involving the weighing of
long with an inside diameter of 1.5 mm.
a known area of specimens having a known density is also
9.1.2 Cell Reservoir System, consisting of a glass reservoir
suitable for homogeneous materials.
of sufficient size to contain all the mercury required in the cell.
9.1.3 Adapters—Solid and hollow adapters for measure-
7. Conditioning
ment of widely varying gas transmission rates. The solid
adapter provides a minimum void volume for slow transmis-
7.1 Standard Conditioning—Condition all test specimens at
sion rates. The hollow adapter increases the void volume by
23 6 2°C in a desiccator over calcium chloride or other
about a factor of eight for faster transmission rates.
suitable desiccant for not less than 48 h prior to test in
accordance with Practice D618, for those tests where condi-
tioning is required. In cases of disagreement, the tolerances
shall be 61°C.
The sole source of supply of the apparatus (Dow gas transmission cell) known
to the committee at this time is Custom Scientific Instruments, Inc., Whippany, NJ.
7.2 Alternative Conditioning—Alternatives to 7.1 may be
If you are aware of alternative suppliers, please provide this information toASTM
used for conditioning the specimens provided that these
International Headquarters. Your comments will receive careful consideration at a
conditions are described in the report. meeting of the responsible technical committee, which you may attend.
´1
D1434 − 82 (2009)
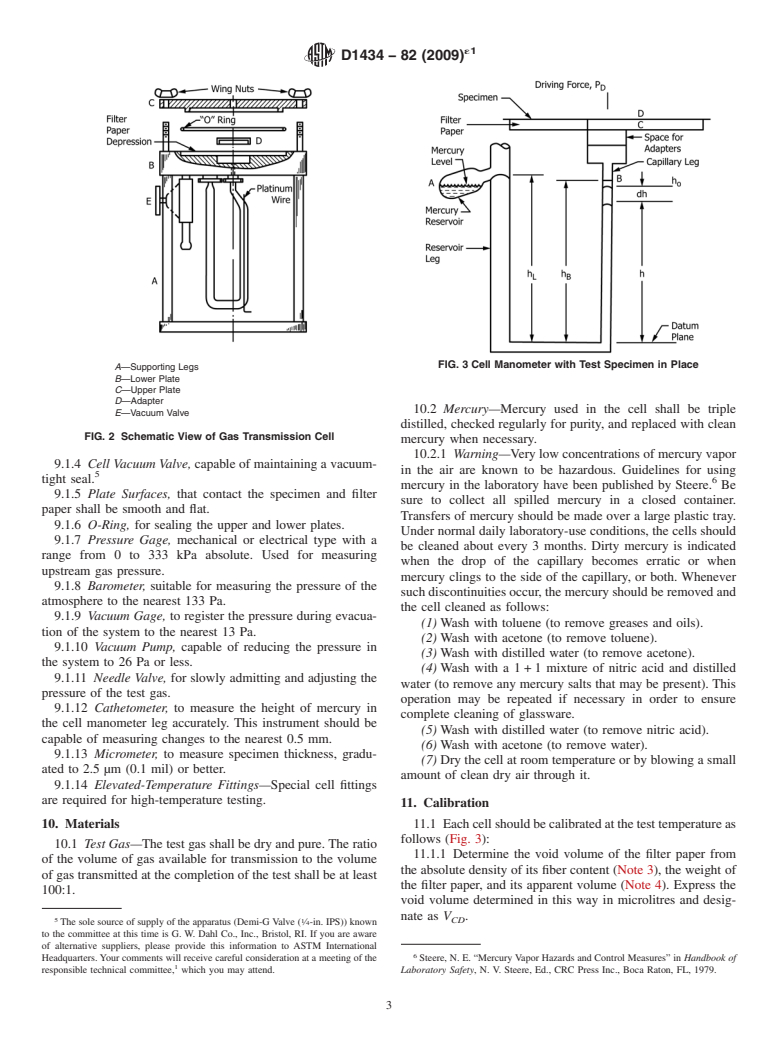
FIG. 3 Cell Manometer with Test Specimen in Place
A—Supporting Legs
B—Lower Plate
C—Upper Plate
D—Adapter
10.2 Mercury—Mercury used in the cell shall be triple
E—Vacuum Valve
distilled, checked regularly for purity, and replaced with clean
FIG. 2 Schematic View of Gas Transmission Cell
mercury when necessary.
10.2.1 Warning—Very low concentrations of mercury vapor
9.1.4 Cell Vacuum Valve, capable of maintaining a vacuum-
in the air are known to be hazardous. Guidelines for using
tight seal. 6
mercury in the laboratory have been published by Steere. Be
9.1.5 Plate Surfaces, that contact the specimen and filter
sure to collect all spilled mercury in a closed container.
paper shall be smooth and flat.
Transfers of mercury should be made over a large plastic tray.
9.1.6 O-Ring, for sealing the upper and lower plates.
Under normal daily laboratory-use conditions, the cells should
9.1.7 Pressure Gage, mechanical or electrical type with a
be cleaned about every 3 months. Dirty mercury is indicated
range from 0 to 333 kPa absolute. Used for measuring
when the drop of the capillary becomes erratic or when
upstream gas pressure.
mercury clings to the side of the capillary, or both. Whenever
9.1.8 Barometer, suitable for measuring the pressure of the
suchdiscontinuitiesoccur,themercuryshouldberemovedand
atmosphere to the nearest 133 Pa.
the cell cleaned as follows:
9.1.9 Vacuum Gage, to register the pressure during evacua-
(1)Wash with toluene (to remove greases and oils).
tion of the system to the nearest 13 Pa.
(2)Wash with acetone (to remove toluene).
9.1.10 Vacuum Pump, capable of reducing the pressure in
(3)Wash with distilled water (to remove acetone).
the system to 26 Pa or less.
(4)Wash with a 1+1 mixture of nitric acid and distilled
9.1.11 Needle Valve, for slowly admitting and adjusting the
water (to remove any mercury salts that may be present). This
pressure of the test gas.
operation may be repeated if necessary in order to ensure
9.1.12 Cathetometer, to measure the height of mercury in
complete cleaning of glassware.
the cell manometer leg accurately. This instrument should be
(5)Wash with distilled water (to remove nitric acid).
capable of measuring changes to the nearest 0.5 mm.
(6)Wash with acetone (to remove water).
9.1.13 Micrometer, to measure specimen thickness, gradu-
(7)Dry the cell at room temperature or by blowing a small
ated to 2.5 µm (0.1 mil) or better.
amount of clean dry air through it.
9.1.14 Elevated-Temperature Fittings—Special cell fittings
are required for high-temperature testing.
11. Calibration
10. Materials 11.1 Eachcellshouldbecalibratedatthetesttemperatureas
follows (Fig. 3):
10.1 Test Gas—The test gas shall be dry and pure.The ratio
11.1.1 Determine the void volume of the filter paper from
of the volume of gas available for transmission to the volume
the absolute density of its fiber content (Note 3), the weight of
of gas transmitted at the completion of the test shall be at least
the filter paper, and its apparent volume (Note 4). Express the
100:1.
void volume determined in this way in microlitres and desig-
nate as V .
1 CD
The sole source of supply of the apparatus (Demi-G Valve ( ⁄4-in. IPS)) known
to the committee at this time is G. W. Dahl Co., Inc., Bristol, RI. If you are aware
of alternative suppliers, please provide this information to ASTM International
Headquarters.Your comments will receive careful consideration at a meeting of the Steere, N. E. “Mercury Vapor Hazards and Control Measures” in Handbook of
responsible technical committee, which you may attend. Laboratory Safety, N. V. Steere, Ed., CRC Press Inc., Boca Raton, FL, 1979.
´1
D1434 − 82 (2009)
NOTE 3—Any high-grade, medium-retention qualitative nonashing
cellulosic filter paper, 90 mm in diameter will be satisfactory for this
purpose. Cellulose fiber has an approximate density of 1.45 g/mL.
NOTE 4—The apparent volume may be calculated from the thickness
and diameter of the filter paper.
11.1.2 Determine the volume of the cell manometer leg
from B to C,Fig. 3, by mercury displacement. (Since the void
volume of the adapters is included in this part of the
calibration, the volume from B to C should be determined
twice, once with the solid adapter in place, and once with the
hollow.)This volume is obtained by dividing the weight of the
mercury displaced by its density (Note 5). Determine this
volume to nearest 1 µL and designate as V .
BC
NOTE 5—The density of mercury at 23°C is 13.54 g/mL.
11.1.3 Determine the volume, in microlitres, of the cell
manometer leg from A to B, Fig. 3, by mercury displacement.
Determine the average cross-sectional area of the capillary by
dividingthisvolumebythelength(expressedtothenearest0.1
FIG. 4 Component Arrangement of Gas Transmission Equipment
mm) from A to B.Determine this area to the nearest 0.01 mm
and designate as a .
c
11.1.4 Determine the area of the filter paper cavity to the
12.6 Locate the O-ring on the upper plate; then carefully
nearest 1 mm . Designate this area as A, the area of transmis-
position this plate over the specimen and fix the plate with
sion.
uniform pressure to ensure a vacuum-tight seal.
11.1.5 Pourthemercuryfromthereservoirintothemanom-
12.7 Connect the line in which the test gas will be subse-
eterofthecellbycarefullytippingthecell.Recordthedistance
quently admit
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D1434–82 (Reapproved 2003) Designation:D1434–82 (Reapproved
´1
2009)
Standard Test Method for
Determining Gas Permeability Characteristics of Plastic Film
and Sheeting
This standard is issued under the fixed designation D1434; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Units information was revised editorially in May 2009.
1. Scope
1.1 This test method covers the estimation of the steady-state rate of transmission of a gas through plastics in the form of film,
sheeting, laminates, and plastic-coated papers or fabrics. This test method provides for the determination of (1) gas transmission
rate (GTR), (2) permeance, and, in the case of homogeneous materials, (3) permeability.
1.2 Two procedures are provided:
1.2.1 Procedure M— Manometric.
1.2.2 Procedure V— Volumetric.
1.3The values stated in SI units are to be regarded as the standard.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D618 Practice for Conditioning Plastics for Testing
D1898 Practice for Sampling of Plastics
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 gas transmission rate, GTR— the quantity of a given gas passing through a unit of the parallel surfaces of a plastic film
inunittimeundertheconditionsoftest.TheSIunitofGTRis1mol/(m ·s).Thetestconditions,includingtemperatureandpartial
pressure of the gas on both sides of the film, must be stated. Other factors, such as relative humidity and hydrostatic pressure, that
influence the transport of the gas must also be stated. The inch-pound unit of GTR, a commonly used unit of GTR, is 1 mL
(STP)/(m ·d) at a pressure differential of one atmosphere.
3.1.2 permeance, P—the ratio of the gas transmission rate to the difference in partial pressure of the gas on the two sides of
the film. The SI unit of permeance is 1 mol/ (m ·s·Pa). The test conditions (see 5.1) must be stated.
3.1.3 permeability, P—the product of the permeance and the thickness of a film. The permeability is meaningful only for
homogeneous materials, in which it is a property characteristic of the bulk material. This quantity should not be used unless the
constancy of the permeability has been verified using several different thicknesses of the material. The SI unit of P is 1
mol/(m·s·Pa). The test conditions (see 3.1) must be stated.
NOTE 1—One millilitre (STP) is 44.62 µmol, one atmosphere is 0.1013 MPa, and one day is 86.4 310 s. GTR in SI units is obtained by multiplying
−10
the value in inch-pound units by 5.160 310 . Additional units and conversions are shown in Appendix X1.
3.1.4 steady state—thestateattainedwhentheamountofgasabsorbedinthefilmisinequilibriumwiththefluxofgasthrough
the film. For Method V, this is obtained when the GTR is constant.
This test method is under the jurisdiction ofASTM Committee F02 on Flexible Barrier MaterialsPackaging and is the direct responsibility of Subcommittee F02.10 on
Permeation.
Current edition approved July 30, 1982. Published November 1982. Originally published as D1434–56T. Last previous edition D1434–75.
Current edition approved May 1, 2009. Published May 2009. Originally approved in 1956. Last previous edition approved in 2003 as D1434–82(2003).
ForreferencedASTMstandards,visittheASTMwebsite,www.astm.org,orcontactASTMCustomerServiceatservice@astm.org.ForAnnualBookofASTMStandards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Withdrawn.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
D1434–82 (2009)
4. Summary of Test Method
4.1 The sample is mounted in a gas transmission cell so as to form a sealed semibarrier between two chambers. One chamber
contains the test gas at a specific high pressure, and the other chamber, at a lower pressure, receives the permeating gas. Either
of the following procedures is used:
4.1.1 Procedure M— In Procedure M the lower pressure chamber is initially evacuated and the transmission of the gas through
the test specimen is indicated by an increase in pressure.
4.1.2 Procedure V— In ProcedureVthe lower pressure chamber is maintained near atmospheric pressure and the transmission
of the gas through the test specimen is indicated by a change in volume.
5. Significance and Use
5.1 Thesemeasurementsgivesemiquantitativeestimatesforthegastransmissionofsinglepuregasesthroughfilmandsheeting.
Correlation of measured values with any given use, such as packaged contents protection, must be determined by experience.The
gas transmission rate is affected by conditions not specifically provided for in these tests, such as moisture content (Note 2),
plasticizer content, and nonhomogeneities. These tests do not include any provision for testing seals that may be involved in
packaging applications.
NOTE 2—The tests are run using gas with 0% moisture changes.
5.2 Interlaboratory testing has revealed that permeances measured by these procedures exhibit a strong dependence on the
procedure being used, as well as on the laboratory performing the testing.Agreement with other methods is sometimes poor and
maybematerial-dependent.Thematerialsbeingtestedoftenaffectthebetween-laboratoryprecision.Thecausesofthesevariations
are not known at this time. It is suggested that this method not be used for referee purposes unless purchaser and seller can both
establish that they are measuring the same quantity to a mutually agreed upon level of precision.
5.3 Use of the permeability coefficient (involving conversion of the gas transmission rate to a unit thickness basis) is not
recommended unless the thickness-to-transmission rate relationship is known from previous studies. Even in essentially
homogeneous structures, variations in morphology (as indicated, for example, by density) and thermal history may influence
permeability.
6. Test Specimen
6.1 The test specimen shall be representative of the material, free of wrinkles, creases, pinholes, and other imperfections, and
shall be of uniform thickness. The test specimen shall be cut to an appropriate size (generally circular) to fit the test cell.
6.2 The thickness of the specimen shall be measured to the nearest 2.5 µm (0.1 mil) with a calibrated dial gage (or equivalent)
at a minimum of five points distributed over the entire test area. Maximum, minimum, and average values should be recorded.An
alternative measure of thickness involving the weighing of a known area of specimens having a known density is also suitable for
homogeneous materials.
7. Conditioning
7.1 Standard Conditioning—Condition all test specimens at 23 6 2°C (73.4 6 3.6°F) in a desiccator over calcium chloride or
other suitable desiccant for not less than 48 h prior to test in accordance with Practice D618, for those tests where conditioning
is required. In cases of disagreement, the tolerances shall be 6 1°C (6 1.8°F). 61°C.
7.2 Alternative Conditioning—Alternatives to 7.1 may be used for conditioning the specimens provided that these conditions
are described in the report.
8. Sampling
8.1 The techniques used in sampling a batch of material to be tested by these procedures must depend upon the kind of
information that is sought. Care should be taken to ensure that samples represent conditions across the width and along the length
of rolls of film. Practice D1898 provides guidelines for deciding what procedures to use in sampling a batch of material. Enough
specimens must be tested to ensure that the information obtained is representative of the batch or other lot size being tested.
PROCEDURE M
(Pressure changes in the manometric cell may be determined by either visual or automatic recording.)
MANOMETRIC VISUAL DETERMINATION
9. Apparatus
9.1 The apparatus shown in Fig. 1 and Fig. 2 consists of the following items:
The Dow gas transmission cell supplied by Custom Scientific Instruments, Inc., Whippany, NJ, has been found satisfactory for this purpose.
´1
D1434–82 (2009)
FIG. 1 Manometric Gas Transmission Cell
A—Supporting Legs
B—Lower Plate
C—Upper Plate
D—Adapter
E—Vacuum Valve
FIG. 2 Schematic View of Gas Transmission Cell
9.1.1 CellManometerSystem—Thecalibratedcellmanometerleg,whichindicatesthepressureoftransmittedgas,shallconsist
of precision-bore glass capillary tubing at least 65 mm long with an inside diameter of 1.5 mm.
9.1.2 Cell Reservoir System, consisting of a glass reservoir of sufficient size to contain all the mercury required in the cell.
9.1.3 Adapters—Solid and hollow adapters for measurement of widely varying gas transmission rates. The solid adapter
provides a minimum void volume for slow transmission rates. The hollow adapter increases the void volume by about a factor of
eight for faster transmission rates.
9.1.4 Cell Vacuum Valve, capable of maintaining a vacuum-tight seal.
9.1.5 Plate Surfaces, that contact the specimen and filter paper shall be smooth and flat.
The sole source of supply of the apparatus (Dow gas transmission cell) known to the committee at this time is Custom Scientific Instruments, Inc., Whippany, NJ. If
you are aware of alternative suppliers, please provide this information toASTM International Headquarters. Your comments will receive careful consideration at a meeting
of the responsible technical committee, which you may attend.
The Demi-G Valve ( ⁄4-in. IPS) manufactured by G. W. Dahl Co., Inc., Bristol, RI, or a precision-ground glass stopcock, meets this requirement.
The sole source of supply of the apparatus (Demi-G Valve ( ⁄4-in. IPS)) known to the committee at this time is G. W. Dahl Co., Inc., Bristol, RI. If you are aware of
alternative suppliers, please provide this information toASTM International Headquarters.Your comments will receive careful consideration at a meeting of the responsible
technical committee, which you may attend.
´1
D1434–82 (2009)
9.1.6 O-Ring, for sealing the upper and lower plates.
9.1.7 Pressure Gage, mechanical or electrical type with a range from 0 to 333 kPa absolute. Used for measuring upstream gas
pressure.
9.1.8 Barometer, suitable for measuring the pressure of the atmosphere to the nearest 133 Pa.
9.1.9 Vacuum Gage, to register the pressure during evacuation of the system to the nearest 13 Pa.
9.1.10 Vacuum Pump, capable of reducing the pressure in the system to 26 Pa or less.
9.1.11 Needle Valve, for slowly admitting and adjusting the pressure of the test gas.
9.1.12 Cathetometer, to measure the height of mercury in the cell manometer leg accurately.This instrument should be capable
of measuring changes to the nearest 0.5 mm.
9.1.13 Micrometer, to measure specimen thickness, graduated to 2.5 µm (0.1 mil) or better.
9.1.14 Elevated-Temperature Fittings —Special cell fittings are required for high-temperature testing.
10. Materials
10.1 Test Gas—The test gas shall be dry and pure. The ratio of the volume of gas available for transmission to the volume of
gas transmitted at the completion of the test shall be at least 100:1.
10.2 Mercury—Mercury used in the cell shall be triple distilled, checked regularly for purity, and replaced with clean mercury
when necessary.
10.2.1 Warning—Verylowconcentrationsofmercuryvaporintheairareknowntobehazardous.Guidelinesforusingmercury
in the laboratory have been published by Steere. Be sure to collect all spilled mercury in a closed container.Transfers of mercury
should be made over a large plastic tray. Under normal daily laboratory-use conditions, the cells should be cleaned about every
3 months. Dirty mercury is indicated when the drop of the capillary becomes erratic or when mercury clings to the side of the
capillary, or both. Whenever such discontinuities occur, the mercury should be removed and the cell cleaned as follows:
(1) Wash with toluene (to remove greases and oils).
(2) Wash with acetone (to remove toluene).
(3) Wash with distilled water (to remove acetone).
(4) Wash with a 1+1 mixture of nitric acid and distilled water (to remove any mercury salts that may be present). This
operation may be repeated if necessary in order to ensure complete cleaning of glassware.
(5) Wash with distilled water (to remove nitric acid).
(6) Wash with acetone (to remove water).
(7) Dry the cell at room temperature or by blowing a small amount of clean dry air through it.
11. Calibration
11.1 Each cell should be calibrated at the test temperature as follows (Fig. 3):
11.1.1 Determine the void volume of the filter paper from the absolute density of its fiber content (Note 3), the weight of the
filterpaper,anditsapparentvolume(Note4).ExpressthevoidvolumedeterminedinthiswayinmicrolitresanddesignateasV .
CD
Steere, N. E. “Mercury Vapor Hazards and Control Measures” in Handbook of Laboratory Safety, N. V. Steere, Ed., CRC Press Inc., Boca Raton, FL, 1979.
FIG. 3 Cell Manometer with Test Specimen in Place
´1
D1434–82 (2009)
NOTE 3—Any high-grade, medium-retention qualitative nonashing cellulosic filter paper, 90 mm in diameter will be satisfactory for this purpose.
Cellulose fiber has an approximate density of 1.45 g/mL.
NOTE 4—The apparent volume may be calculated from the thickness and diameter of the filter paper.
11.1.2 Determine the volume of the cell manometer leg from B to C, Fig. 3, by mercury displacement. (Since the void volume
of the adapters is included in this part of the calibration, the volume from B to C should be determined twice, once with the solid
adapterinplace,andoncewiththehollow.)Thisvolumeisobtainedbydividingtheweightofthemercurydisplacedbyitsdensity
(Note 5). Determine this volume to nearest 1 µL and designate as V .
BC
NOTE 5—The density of mercury at 23°C is 13.54 g/mL.
11.1.3 Determinethevolume,inmicrolitres,ofthecellmanometerlegfromAtoB,Fig.3,bymercurydisplacement.
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.