ASTM F2580-18
(Practice)Standard Practice for Evaluation of Modular Connection of Proximally Fixed Femoral Hip Prosthesis
Standard Practice for Evaluation of Modular Connection of Proximally Fixed Femoral Hip Prosthesis
SIGNIFICANCE AND USE
4.1 This practice can be used to describe the effects of materials, manufacturing, and design variables on the fatigue performance of metallic femoral hip prostheses subject to cyclic loading for large numbers of cycles.
4.2 The loading of femoral hip designs in vivo will, in general, differ from the loading defined in this practice. The results obtained here cannot be used to directly predict in vivo performance. However, this practice is designed to allow for comparisons between the fatigue performance of different metallic femoral hip designs when tested under similar conditions.
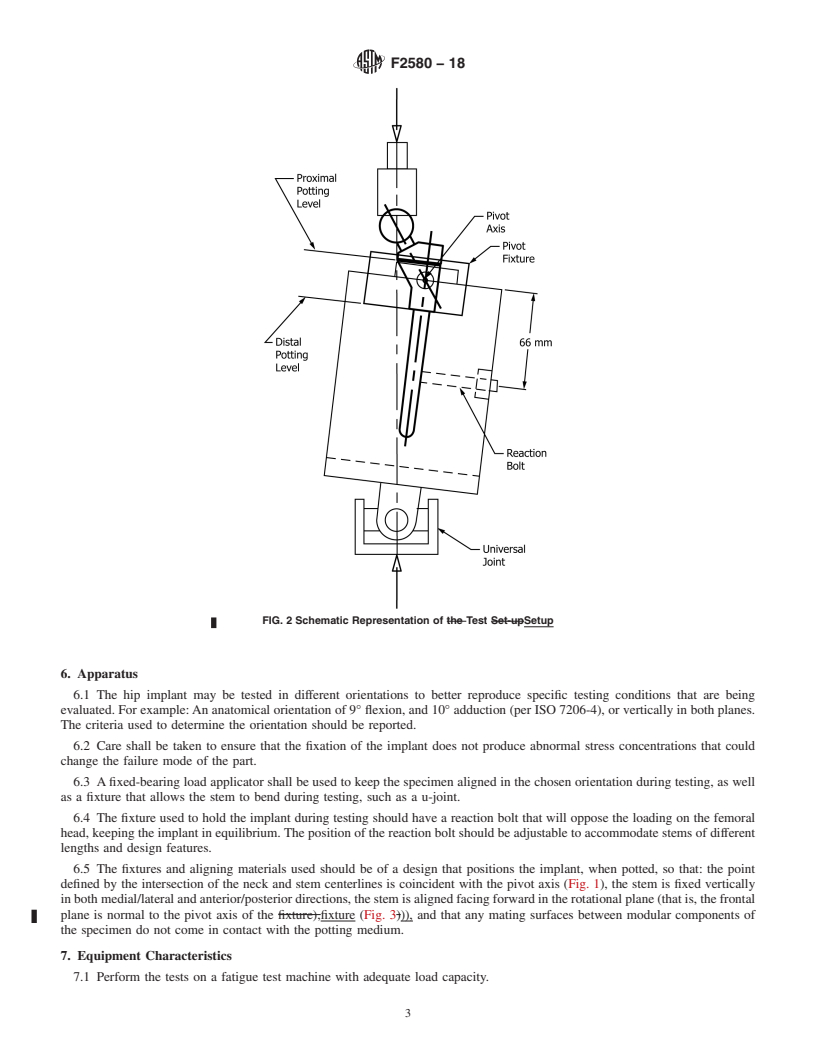
4.3 In order for fatigue data on femoral hip prostheses to be comparable, reproducible, and capable of being correlated among laboratories, it is essential that uniform procedures be established.
SCOPE
1.1 This practice covers a procedure for the fatigue testing of metallic femoral hip prostheses used in hip joint replacements. This practice covers the procedures for the performance of fatigue tests on metallic femoral hip stems using a cyclic, constant-amplitude force. It applies to hip prostheses that utilize proximal metaphyseal fixation and are of a modular construct, and it is intended to evaluate the fatigue performance of the modular connections in the metaphyseal filling (that is, proximal body) region of the stem.
1.2 This practice is intended to provide useful, consistent, and reproducible information about the fatigue performance of metallic hip prostheses while held in a proximally fixated manner, with the distal end not held by a potting medium.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2580 − 18
Standard Practice for
Evaluation of Modular Connection of Proximally Fixed
1
Femoral Hip Prosthesis
This standard is issued under the fixed designation F2580; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E467 Practice for Verification of Constant Amplitude Dy-
namic Forces in an Axial Fatigue Testing System
1.1 This practice covers a procedure for the fatigue testing
E468 Practice for Presentation of Constant Amplitude Fa-
of metallic femoral hip prostheses used in hip joint replace-
tigue Test Results for Metallic Materials
ments. This practice covers the procedures for the performance
E1823 Terminology Relating to Fatigue and Fracture Testing
of fatigue tests on metallic femoral hip stems using a cyclic,
3
2.2 ISO Standards:
constant-amplitude force. It applies to hip prostheses that
ISO 7206–4 Implants for surgery -- Partial and total hip joint
utilize proximal metaphyseal fixation and are of a modular
prostheses -- Part 4: Determination of endurance proper-
construct, and it is intended to evaluate the fatigue performance
ties and performance of stemmed femoral components
of the modular connections in the metaphyseal filling (that is,
proximal body) region of the stem.
3. Terminology
1.2 This practice is intended to provide useful, consistent,
3.1 Definitions:
and reproducible information about the fatigue performance of
3.1.1 R value, n—The R value is the ratio of the minimum
metallic hip prostheses while held in a proximally fixated
load to the maximum load.
manner, with the distal end not held by a potting medium.
minimum load
1.3 The values stated in SI units are to be regarded as
R 5
maximum load
standard. No other units of measurement are included in this
3.2 Definitions of Terms Specific to This Standard:
standard.
3.2.1 extraction, n—removal of the femoral hip implant
1.4 This standard does not purport to address all of the
from the femur during surgery.
safety concerns, if any, associated with its use. It is the
3.2.2 extractor hole, n—a hole in the proximal body of the
responsibility of the user of this standard to establish appro-
stem in which an apparatus is placed to remove the implant
priate safety, health, and environmental practices and deter-
from the femur.
mine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accor-
3.2.3 femoral head, n—convex spherical bearing member
dance with internationally recognized principles on standard-
for articulation with the natural acetabulum or prosthetic
ization established in the Decision on Principles for the
acetabulum.
Development of International Standards, Guides and Recom-
3.2.4 femoral head offset, n—the perpendicular distance
mendations issued by the World Trade Organization Technical
from the centerline of the implant stem to the center of the
Barriers to Trade (TBT) Committee.
femoral head.
3.2.5 frontal plane, n—the plane that lies in the medial-
2. Referenced Documents
lateral direction of the implant. Adduction occurs in this plane.
2
2.1 ASTM Standards:
3.2.6 implant centerline, n—the axis that runs vertically
from the proximal body of the implant down the center of the
stem to the distal end.
1
This practice is under the jurisdiction of ASTM Committee F04 on Medical and
3.2.7 pivot axis, n—the center of rotation of the pivot fixture
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.22 on Arthroplasty. (and prosthesis potted within it) within the test fixture setup; its
Current edition approved July 1, 2018. Published September 2018. Originally
location is determined by the intersection of the neck and stem
approved in 2007. Last previous edition approved in 2013 as F2580 – 13. DOI:
centerlines of the prothesis (Figs. 1 and 2).
10.1520/F2580-18.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2580 − 18
FIG. 1 Free Body Diagram of Test Setup
FIG. 2 Schematic Re
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2580 − 13 F2580 − 18
Standard Practice for
Evaluation of Modular Connection of Proximally Fixed
1
Femoral Hip Prosthesis
This standard is issued under the fixed designation F2580; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice covers a procedure for the fatigue testing of metallic femoral hip prostheses used in hip joint replacements.
This practice covers the procedures for the performance of fatigue tests on metallic femoral hip stems using a cyclic,
constant-amplitude force. It applies to hip prostheses that utilize proximal metaphyseal fixation and are of a modular construct, and
it is intended to evaluate the fatigue performance of the modular connections in the metaphyseal filling (that is, proximal body)
region of the stem.
1.2 This practice is intended to provide useful, consistent, and reproducible information about the fatigue performance of
metallic hip prostheses while held in a proximally fixated manner, with the distal end not held by a potting medium.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
E467 Practice for Verification of Constant Amplitude Dynamic Forces in an Axial Fatigue Testing System
E468 Practice for Presentation of Constant Amplitude Fatigue Test Results for Metallic Materials
E1150E1823 Definitions of Terms Terminology Relating to Fatigue and Fracture Testing (Withdrawn 1996)
3
2.2 ISO Standards:
ISO 7206–4 Determination of Endurance Properties of Stemmed Femoral Components with Application of TorsionImplants for
surgery -- Partial and total hip joint prostheses -- Part 4: Determination of endurance properties and performance of stemmed
femoral components
3. Terminology
3.1 Definitions:
3.1.1 R value, n—The R value is the ratio of the minimum load to the maximum load.
minimum load
R 5
maximum load
3.2 Definitions of Terms Specific to This Standard:
3.2.1 extraction—extraction, n—removal of the femoral hip implant from the femur during surgery.
1
This practice is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.22
on Arthroplasty.
Current edition approved Feb. 1, 2013July 1, 2018. Published February 2013September 2018. Originally approved in 2007. Last previous edition approved in 20092013
as F2580 – 09.F2580 – 13. DOI: 10.1520/F2580-13.10.1520/F2580-18.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2580 − 18
3.2.2 extractor hole—hole, n—a hole in the proximal body of the stem in which an apparatus is placed to remove the implant
from the femur.
3.2.3 femoral head—head, n—convex spherical bearing member for articulation with the natural acetabulum or prosthetic
acetabulum.
3.2.4 femoral head offset—offset, n—the perpendicular distance from the centerline of the implant stem to the center of the
femoral head.
3.2.5 frontal plane—plane, n—the plane that lies in the medial-lateral direction of the implant. Adduction occurs in this plane.
3.2.6 implant centerline—centerline, n—the axis that runs vertically from the proximal body of the implant,implant d
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.