ASTM F3474-20
(Practice)Standard Practice for Establishing Exoskeleton Functional Ergonomic Parameters and Test Metrics
Standard Practice for Establishing Exoskeleton Functional Ergonomic Parameters and Test Metrics
SIGNIFICANCE AND USE
2.1 This practice describes what measure should be performed during the near (hours/days), mid (days/weeks), and far (months/years) stages of exoskeleton evaluation (Fig. 1). The functional conditions and metrics with respect to each task method are assessed from the body area(s) impacted by the exoskeleton (for example, upper body, lower body, or both). These may be within as well as distant to the body areas impacted by the exoskeleton (for example, an upper body exoskeleton may have impacts on the trunk and spine). Desired effects as well as unintended encumbrances to the user’s body are important considerations. The evaluation will occur within the context relevant to the end-use application of the exoskeleton’s implementation. This practice pertains to the industry, military, medical, first responders, and recreational domains, but other domains may arise in the future and will need to be considered. Each domain is unique unto itself; however, the task methods and metrics collected may be unique or overlap across any number of user domains.
FIG. 1 Exoskeleton Assessment Decision Tree
2.2 Task methods and their metrics are either administered in a laboratory environment, field environment, or both laboratory and field environments. Where not otherwise specified, patient functional outcome measures and pain are key metrics that should be considered for testing performed in the medical domain. Exoskeleton producers or researchers, or both, may also want to consider different types of imaging such as X-rays, computed tomography (CT) scans, magnetic resonance imaging (MRI), ultrasound, and nuclear medicine imaging. Additionally, exoskeleton producers or researchers, or both, may also wish to carry out neuroimaging such as, but not limited to, structural and functional and diffusion MRI, magnetoencephalography (MEG), electroencephalography (EEG), positron emission tomography (PET), or near infrared spectroscopy (NIRS) to understand the cognitive and neurophysiol...
SCOPE
1.1 This practice provides a recommended approach and a set of options for assessing one or more specific ergonomic parameters with respect to human users of exoskeletons.
1.2 This practice provides functional ergonomic criteria to consider for the design, production, and evaluation of exoskeletons within the domains of industry, military, medical, first responders, and recreational. When designing exoskeletons, natural unassisted human kinematics and kinetics, as well as the resulting strain and fatigue experienced by the user should be salient design parameters. Any changes in the natural unassisted human kinematics and kinetics may impact the exoskeleton’s effectiveness in augmenting user performance. Therefore, the defining principle of this practice is to establish objective measures that can be selected from to assess human kinematics and kinetics, as well as the resulting strain and fatigue experienced by the user within the task context of the exoskeleton’s end use application.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3474 − 20
Standard Practice for
Establishing Exoskeleton Functional Ergonomic Parameters
1
and Test Metrics
This standard is issued under the fixed designation F3474; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope exoskeleton (for example, upper body, lower body, or both).
These may be within as well as distant to the body areas
1.1 This practice provides a recommended approach and a
impacted by the exoskeleton (for example, an upper body
set of options for assessing one or more specific ergonomic
exoskeletonmayhaveimpactsonthetrunkandspine).Desired
parameters with respect to human users of exoskeletons.
effects as well as unintended encumbrances to the user’s body
1.2 This practice provides functional ergonomic criteria to
are important considerations. The evaluation will occur within
consider for the design, production, and evaluation of exoskel-
the context relevant to the end-use application of the exoskel-
etons within the domains of industry, military, medical, first
eton’s implementation. This practice pertains to the industry,
responders, and recreational. When designing exoskeletons,
military, medical, first responders, and recreational domains,
natural unassisted human kinematics and kinetics, as well as
but other domains may arise in the future and will need to be
the resulting strain and fatigue experienced by the user should
considered. Each domain is unique unto itself; however, the
be salient design parameters. Any changes in the natural
task methods and metrics collected may be unique or overlap
unassisted human kinematics and kinetics may impact the
across any number of user domains.
exoskeleton’s effectiveness in augmenting user performance.
Therefore, the defining principle of this practice is to establish
2.2 Task methods and their metrics are either administered
objective measures that can be selected from to assess human
in a laboratory environment, field environment, or both labo-
kinematics and kinetics, as well as the resulting strain and
ratory and field environments. Where not otherwise specified,
fatigue experienced by the user within the task context of the
patient functional outcome measures and pain are key metrics
exoskeleton’s end use application.
that should be considered for testing performed in the medical
1.3 This standard does not purport to address all of the
domain. Exoskeleton producers or researchers, or both, may
safety concerns, if any, associated with its use. It is the
alsowanttoconsiderdifferenttypesofimagingsuchasX-rays,
responsibility of the user of this standard to establish appro-
computed tomography (CT) scans, magnetic resonance imag-
priate safety, health, and environmental practices and deter-
ing (MRI), ultrasound, and nuclear medicine imaging.
mine the applicability of regulatory limitations prior to use.
Additionally, exoskeleton producers or researchers, or both,
1.4 This international standard was developed in accor-
may also wish to carry out neuroimaging such as, but not
dance with internationally recognized principles on standard-
limited to, structural and functional and diffusion MRI, mag-
ization established in the Decision on Principles for the
netoencephalography (MEG), electroencephalography (EEG),
Development of International Standards, Guides and Recom-
positron emission tomography (PET), or near infrared spec-
mendations issued by the World Trade Organization Technical
troscopy (NIRS) to understand the cognitive and neurophysi-
Barriers to Trade (TBT) Committee.
ological impacts that exoskeletons have on the brain.
2. Significance and Use
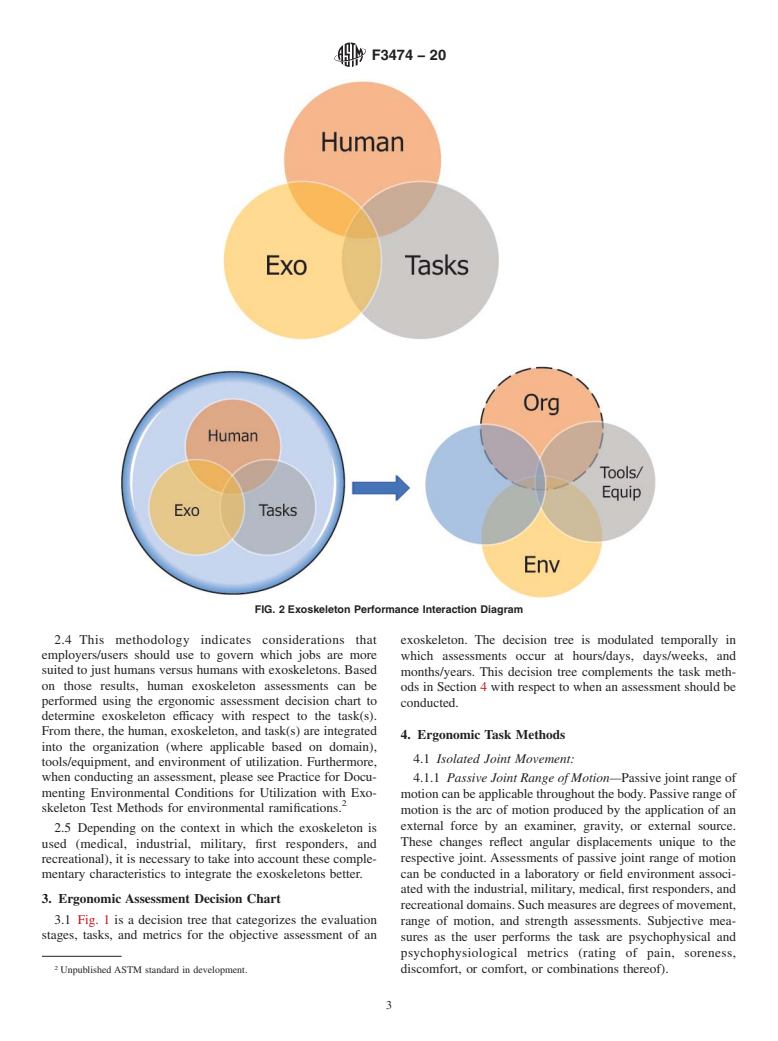
2.3 Fig. 2 is a Venn diagram that portrays the distinct
2.1 This practice describes what measure should be per- interactions that may transpire between the user (human),
formedduringthenear(hours/days),mid(days/weeks),andfar exoskeleton, and task. The interactions are:
(months/years) stages of exoskeleton evaluation (Fig. 1). The
2.3.1 Human—For example, baseline health, physiology,
functional conditions and metrics with respect to each task
and job duty assessment;
method are assessed from the body area(s) impacted by the
2.3.2 Human with an Exoskeleton—For example, fit,
comfort, physical size changes, psychosocial considerations,
1
cognitive load, training level, donning/doffing time, and safety;
This practice is under the jurisdiction of ASTM Committee F48 on Exoskel-
etons and Exosuits and is the direct responsibility of
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.